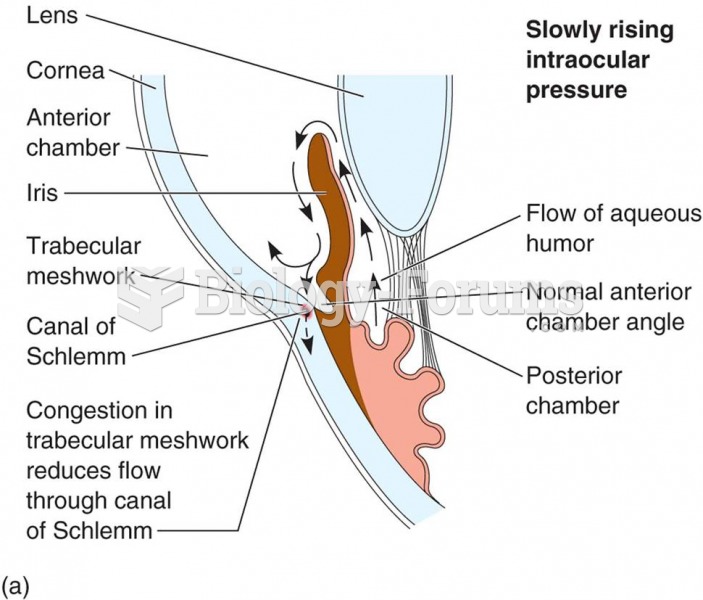
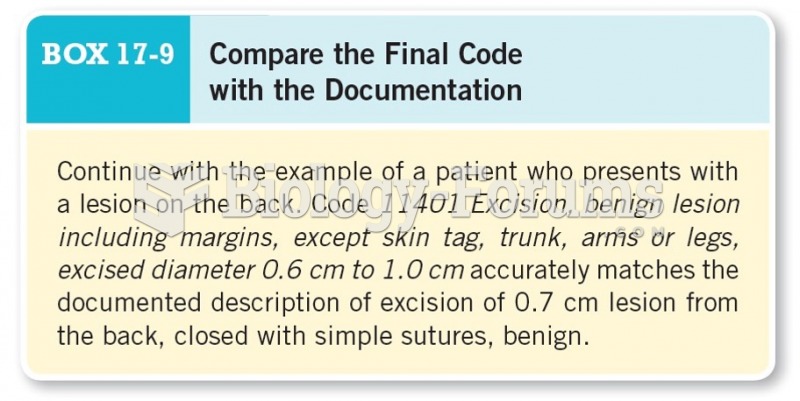
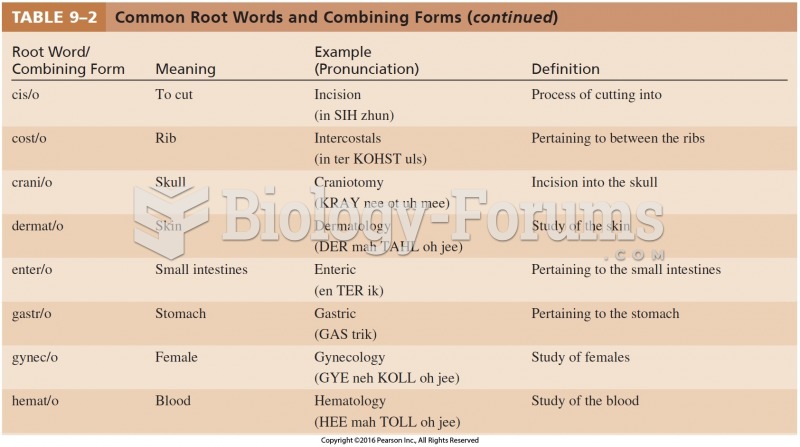
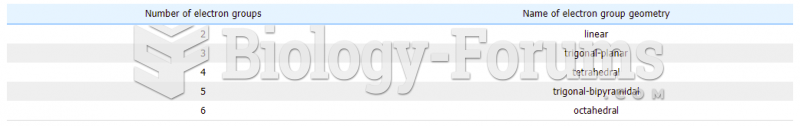
Answer to Question 1
Answer:
Claims should be orderly with important facts that furnish decisive evidence of claims billing or processing. Most computer software systems will have a place for documenting in the claim file pertinent conversations with patients, providers, and/or insurance representatives. If the claim requires more information to be processed, the claims examiner should indicate the disposition of the claim in the notes. It is the medical biller's or claims examiner's responsibility to document the information requested and the date of the request. In addition, the date a follow-up letter is required (in the event that the information is not received within a certain amount of time) should also be included in the documentation.
This documentation enables work to be done on a claim at a future date. Since you may not be the person who follows up on the claim or receives the information requested, be sure that the documentation is understandable and that it provides concise, informative, and pertinent information. Proper documentation should have the following attributes:
a. Professional. Keep personal remarks out of documentation. Keep your information objective. Do not bold, highlight, or otherwise mark any sections, passages, or words of a narrative report or claim form. As innocent as it may seem, a judge, jury, or plaintiff's attorney may see it as singling out, bad faith, discriminatory, or arbitrary.
b. Evidence based. The representations made in documentation must not only be fair and reasonable but must also create a file that shows fairness, reasonableness, and factual accuracy when read by a jury. Correctly record the basis for all coding or claims decisions. Such a practice is a double check to ensure your documentation is objective and your decisions are correct.
c. Language. Words or phrases may be taken out of context and used to give a bad connotation to your documentation. Be particularly aware of words that may have a double meaning, especially if taken out of context. Some programs have built in checklists that help the biller/examiner use specific approved language.
d. Communication with an attorney. If communicating with an attorney representing the claimant, give only initial, factual information. If the attorney requests further information, such as questions concerning the extent and nature of the factual information provided, discuss your response with a member of your company's legal department or a supervisor before responding. All information or printouts of documents provided should be clearly documented in the file.
Answer to Question 2
D