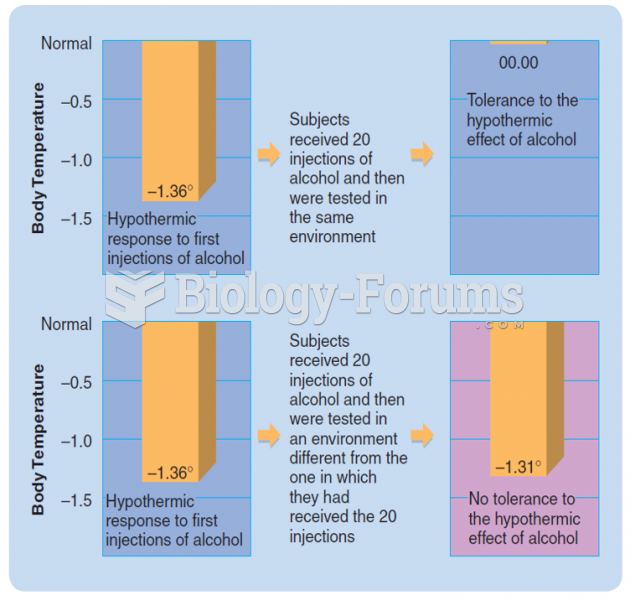
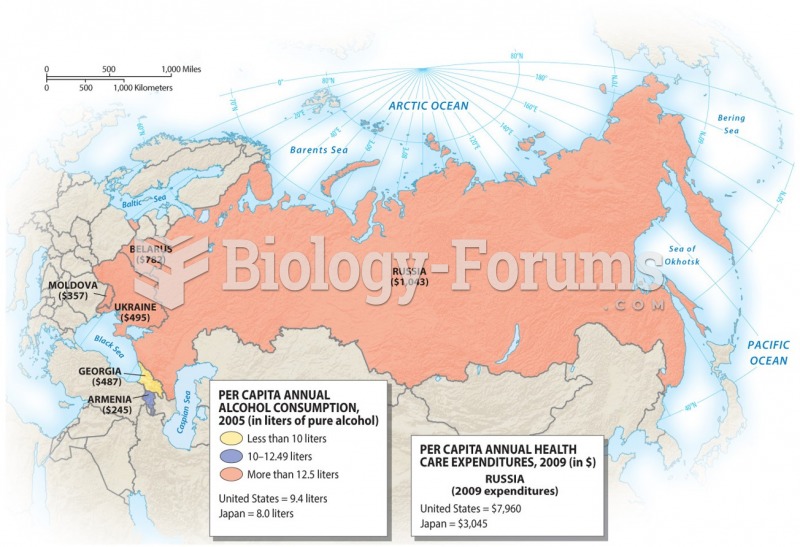
|
|
|
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Cutaneous mucormycosis is a rare fungal infection that has been fatal in at least 29% of cases, and in as many as 83% of cases, depending on the patient's health prior to infection. It has occurred often after natural disasters such as tornados, and early treatment is essential.
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
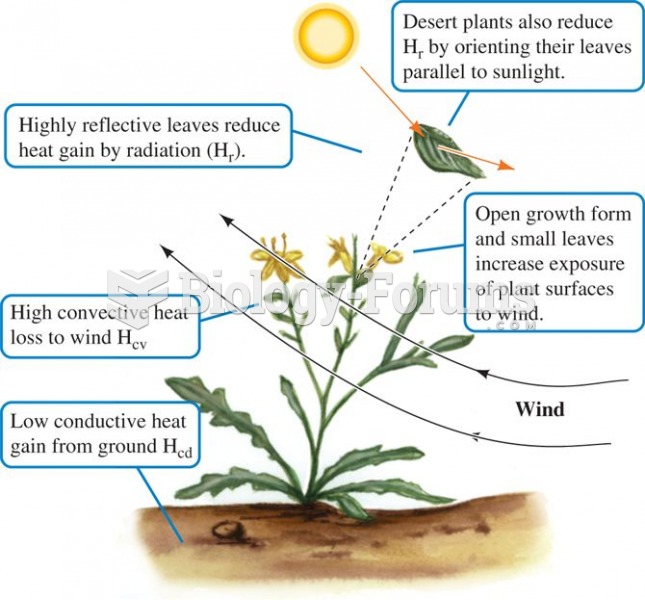 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
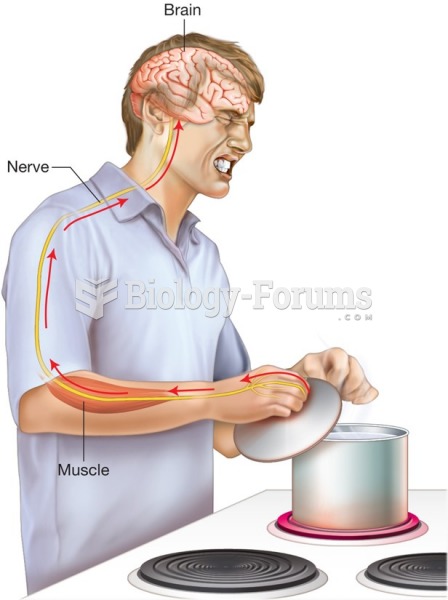 The integumentary and nervous systems work together to recognize specific sensations, such as heat ...
The integumentary and nervous systems work together to recognize specific sensations, such as heat ...