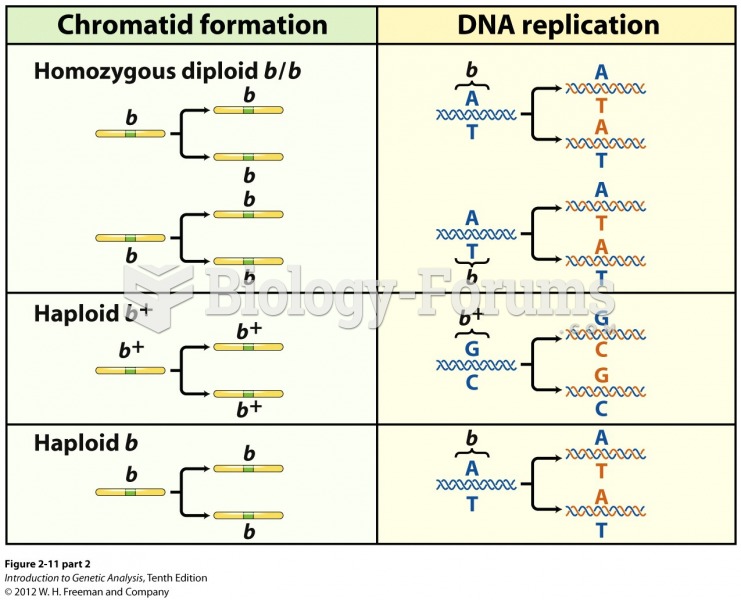
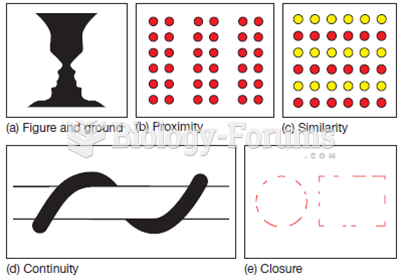
|
|
|
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
The Centers for Disease Control and Prevention has released reports detailing the deaths of infants (younger than 1 year of age) who died after being given cold and cough medications. This underscores the importance of educating parents that children younger than 2 years of age should never be given over-the-counter cold and cough medications without consulting their physicians.
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
 Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
 Effect of pH on drug absorption: (a) A weak acid such as aspirin (ASA) is in a nonionized form in an ...
Effect of pH on drug absorption: (a) A weak acid such as aspirin (ASA) is in a nonionized form in an ...