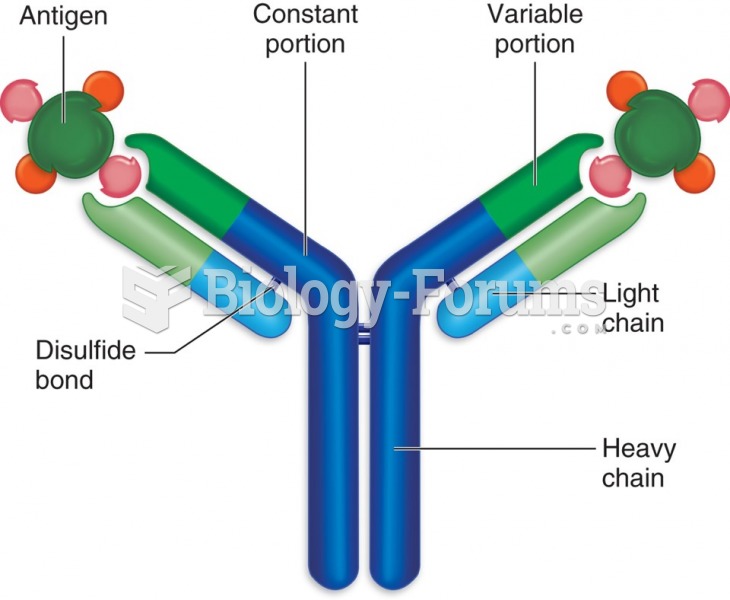
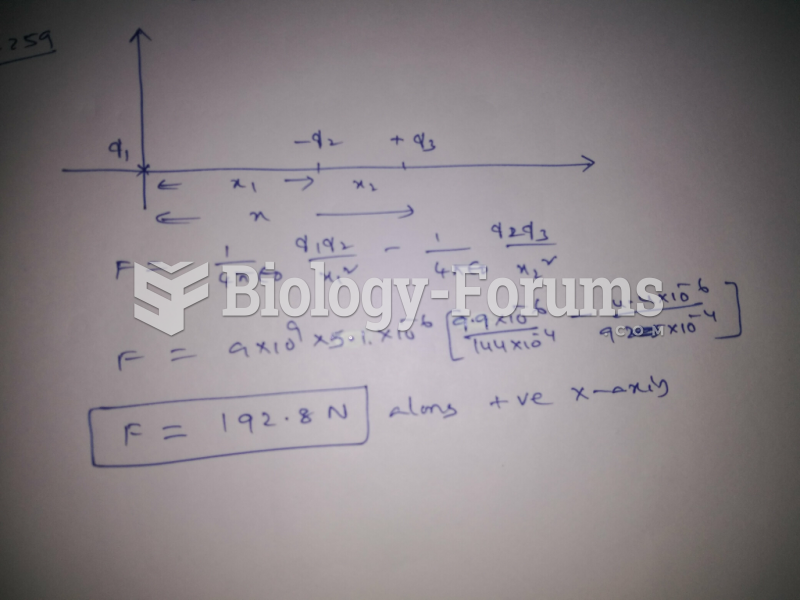
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Pink eye is a term that refers to conjunctivitis, which is inflammation of the thin, clear membrane (conjunctiva) over the white part of the eye (sclera). It may be triggered by a virus, bacteria, or foreign body in the eye. Antibiotic eye drops alleviate bacterial conjunctivitis, and antihistamine allergy pills or eye drops help control allergic conjunctivitis symptoms.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.