|
|
|
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
 Cade and colleagues discovered a way to improve athletic performance and prevent salt and water imba
Cade and colleagues discovered a way to improve athletic performance and prevent salt and water imba
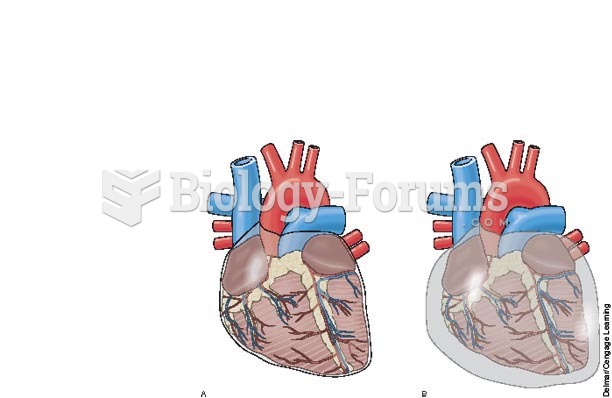 Pericardial effusion: A, normal pericardial sac; B, pericardial sac with excess fluid possibly causi
Pericardial effusion: A, normal pericardial sac; B, pericardial sac with excess fluid possibly causi