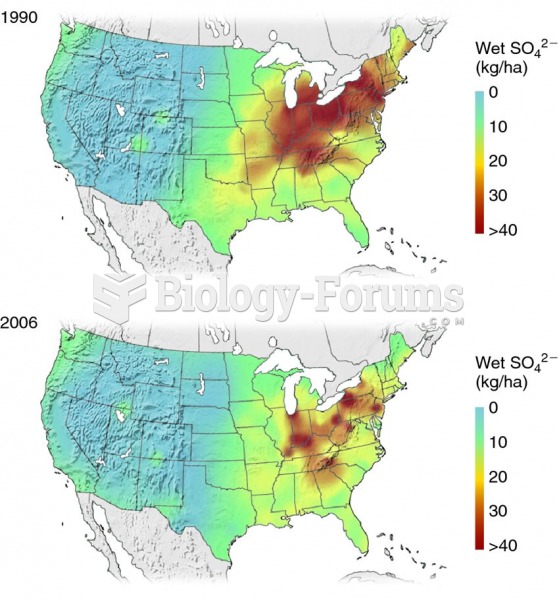
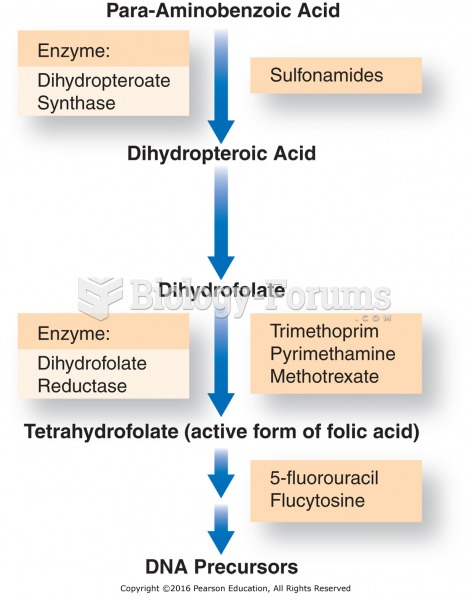
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.