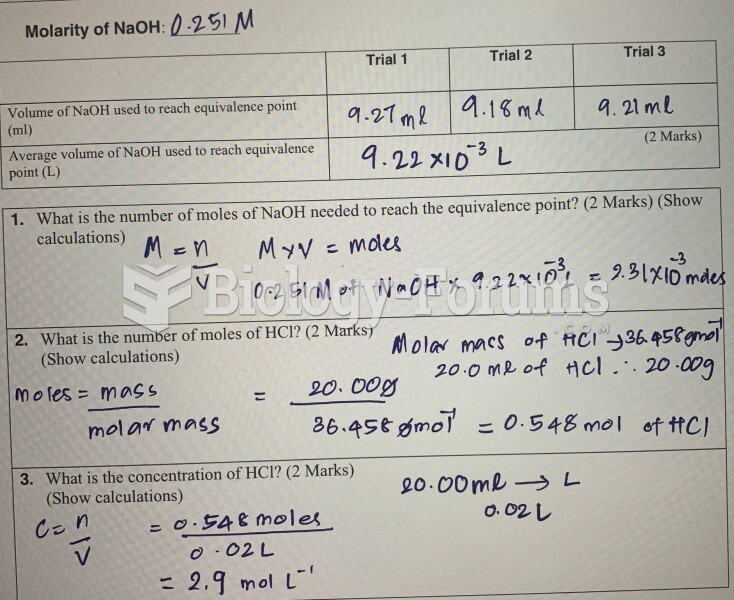
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
Pope Sylvester II tried to introduce Arabic numbers into Europe between the years 999 and 1003, but their use did not catch on for a few more centuries, and Roman numerals continued to be the primary number system.