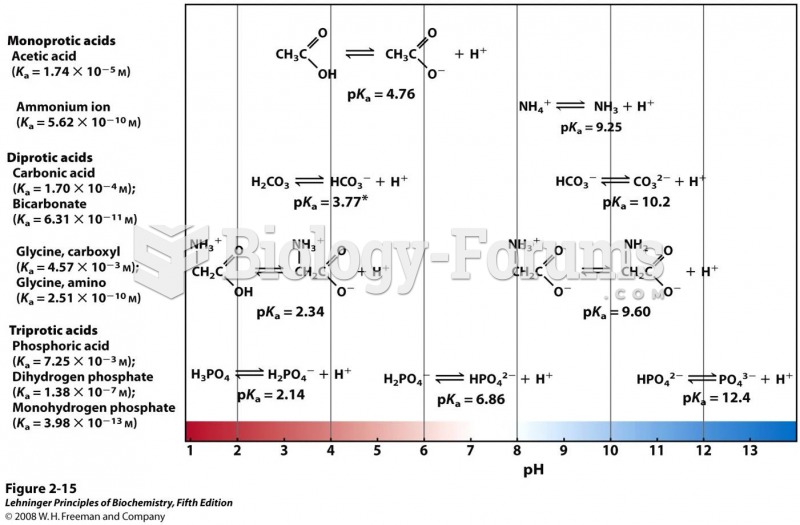
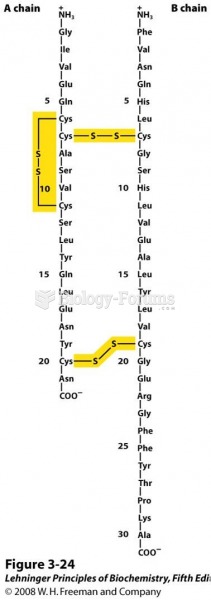
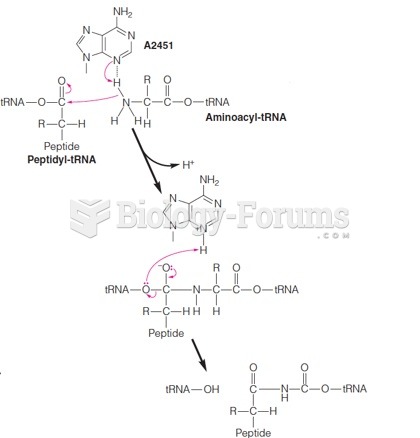
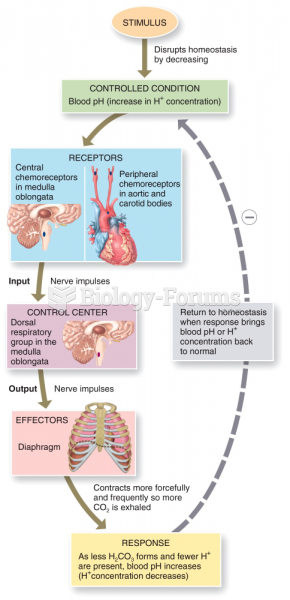
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.