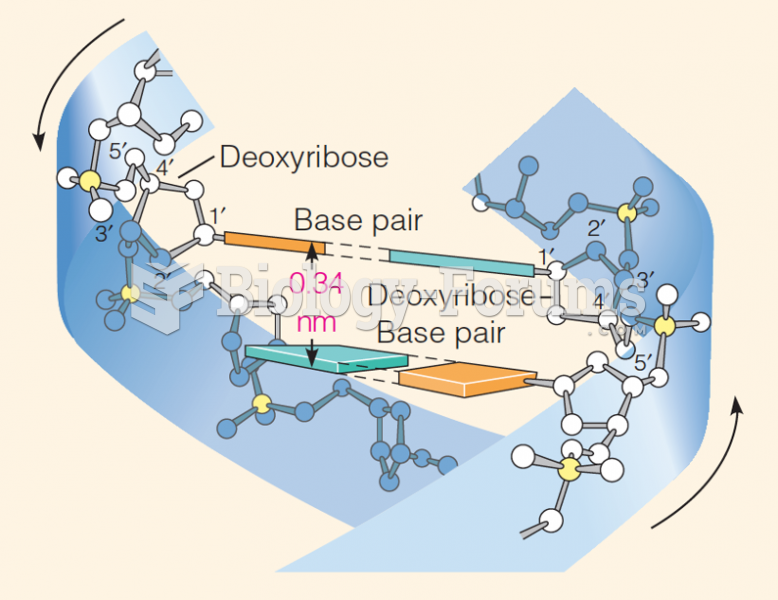
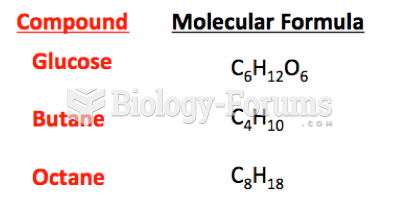
|
|
|
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Never take aspirin without food because it is likely to irritate your stomach. Never give aspirin to children under age 12. Overdoses of aspirin have the potential to cause deafness.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.