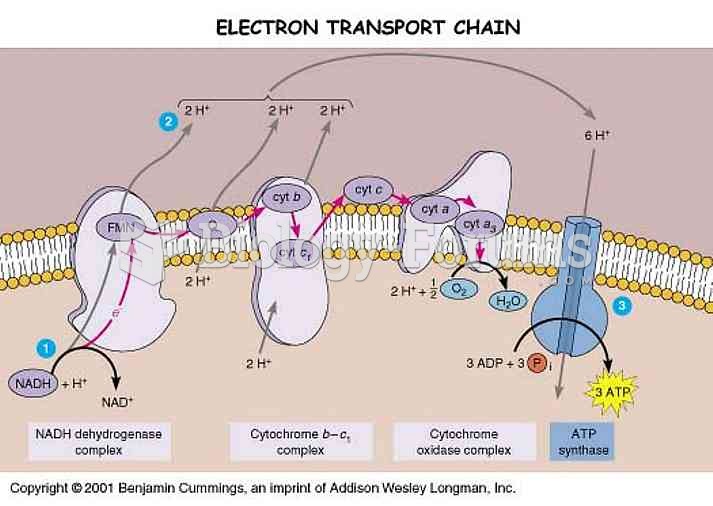
|
|
|
There are approximately 3 million unintended pregnancies in the United States each year.
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.