|
|
|
According to the American College of Allergy, Asthma & Immunology, more than 50 million Americans have some kind of food allergy. Food allergies affect between 4 and 6% of children, and 4% of adults, according to the CDC. The most common food allergies include shellfish, peanuts, walnuts, fish, eggs, milk, and soy.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
People about to have surgery must tell their health care providers about all supplements they take.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
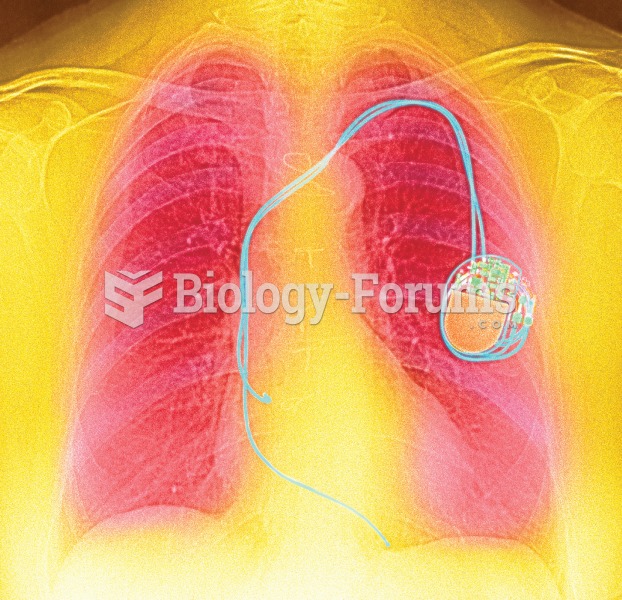 Color enhanced X-ray showing a pacemaker implanted in the left side of the chest and the electrode w
Color enhanced X-ray showing a pacemaker implanted in the left side of the chest and the electrode w
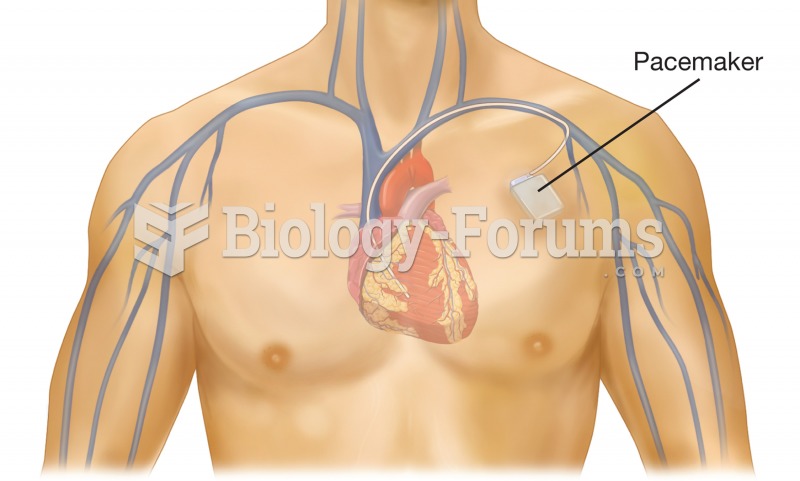 Cardiac pacemaker. The pacemaker device is implanted beneath the skin near the heart, and the electr
Cardiac pacemaker. The pacemaker device is implanted beneath the skin near the heart, and the electr