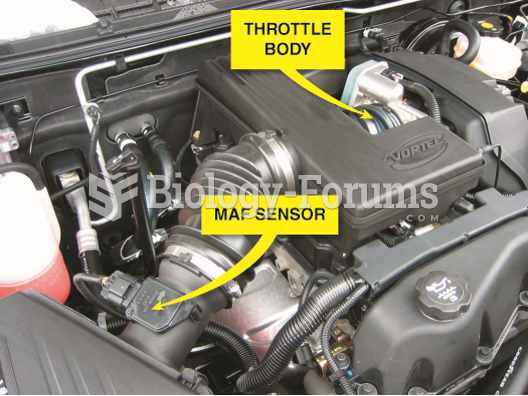
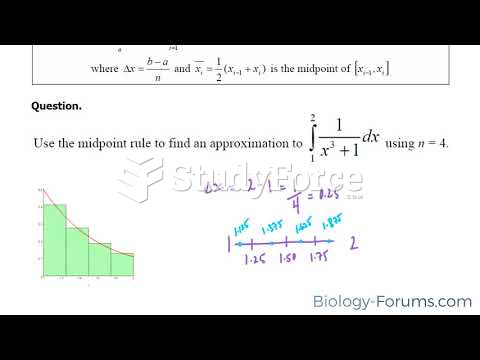
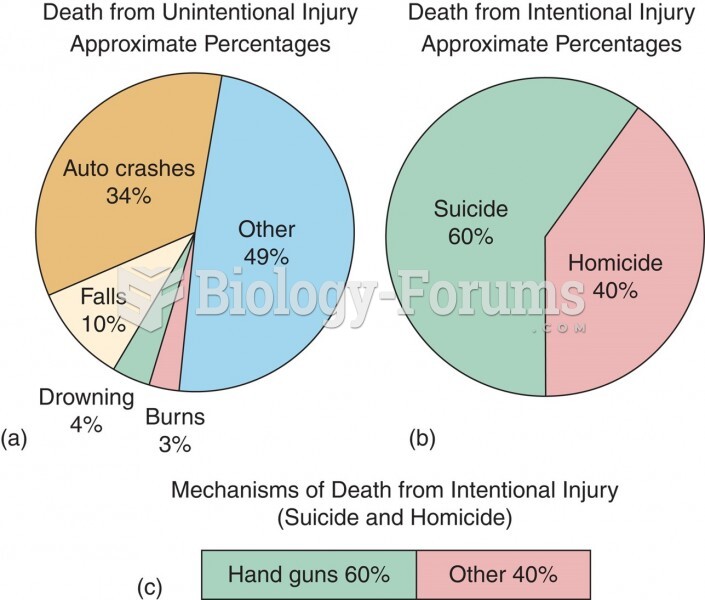
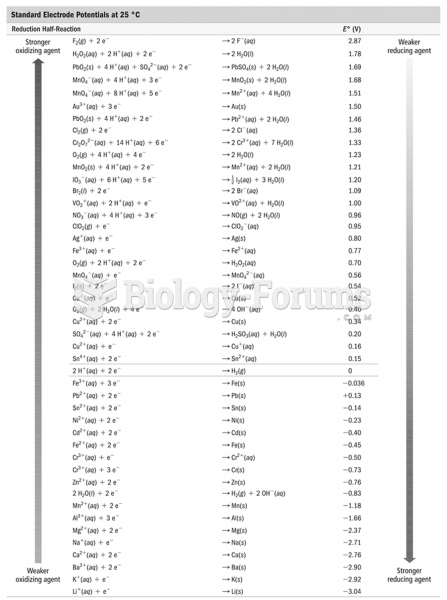
|
|
|
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
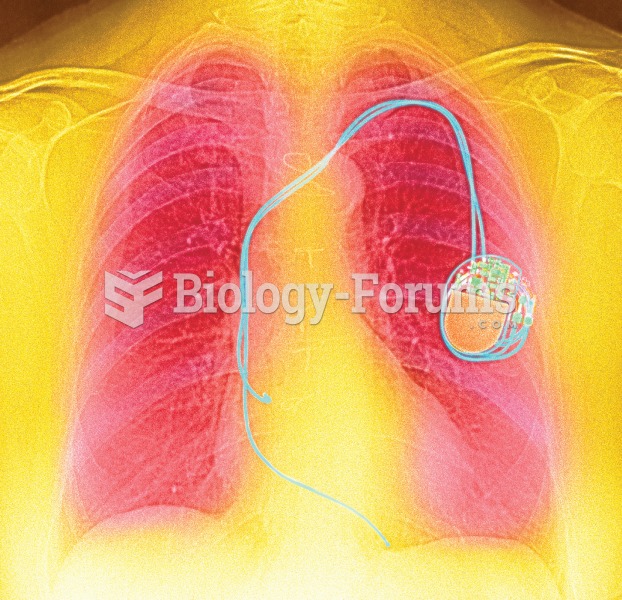 Color enhanced X-ray showing a pacemaker implanted in the left side of the chest and the electrode w
Color enhanced X-ray showing a pacemaker implanted in the left side of the chest and the electrode w
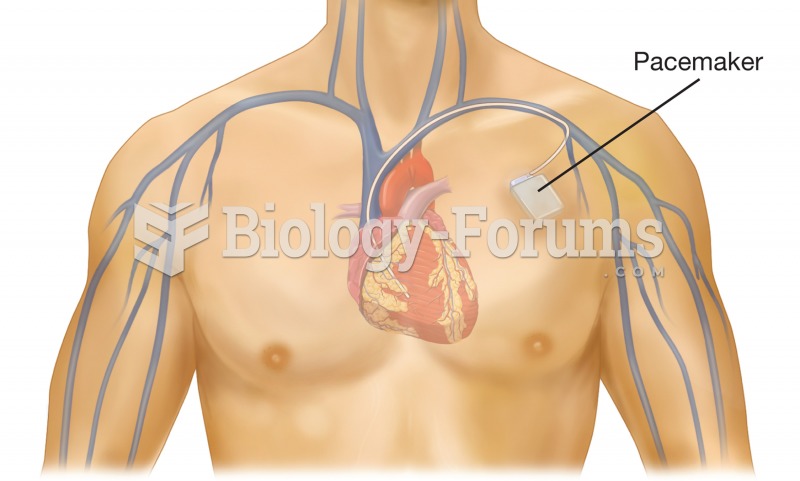 Cardiac pacemaker. The pacemaker device is implanted beneath the skin near the heart, and the electr
Cardiac pacemaker. The pacemaker device is implanted beneath the skin near the heart, and the electr