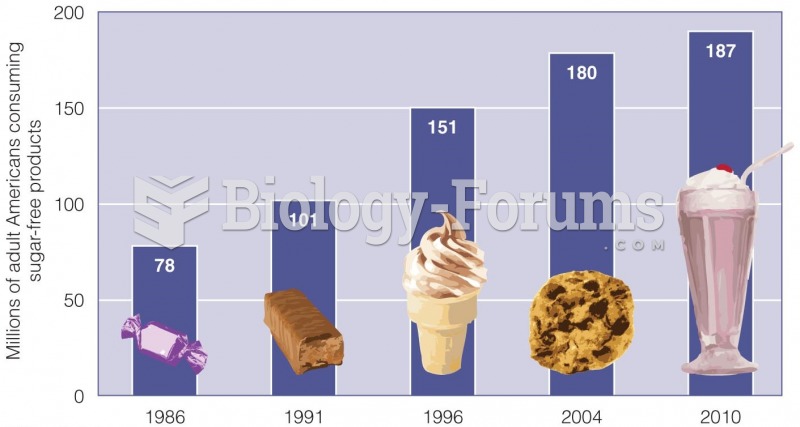
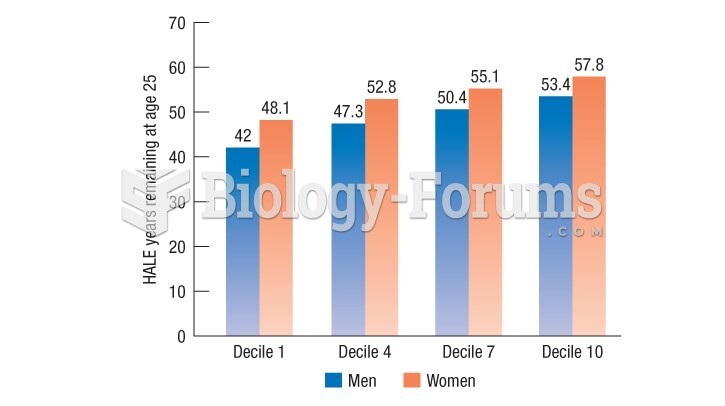
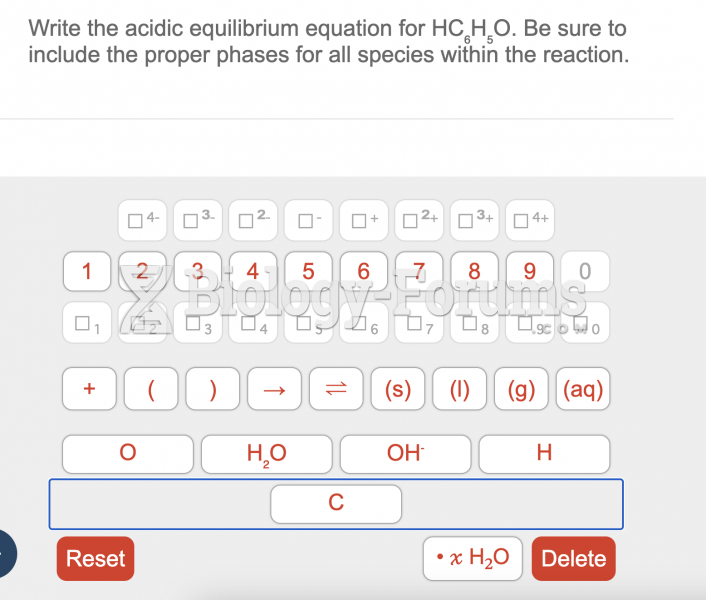
|
|
|
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.