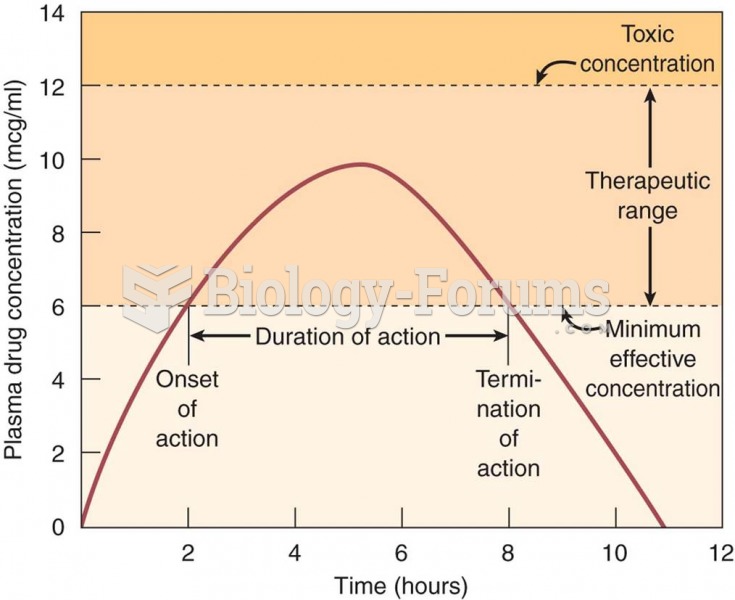
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
Did you know?
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.