|
|
|
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
If all the neurons in the human body were lined up, they would stretch more than 600 miles.



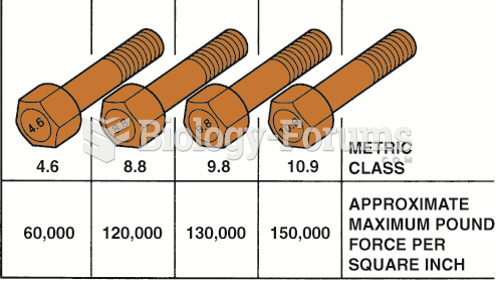

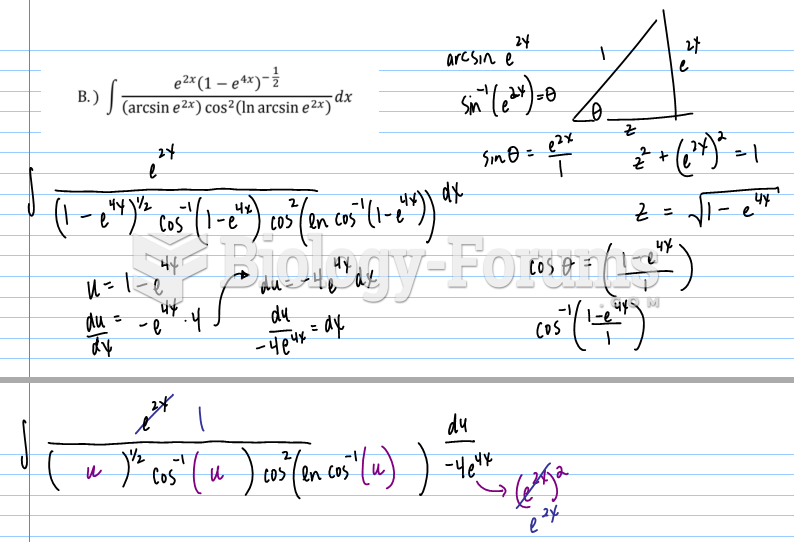
![What is the pH of an aqueous solution if the [H+] = 0.000 000 075 M?](https://biology-forums.com/gallery/43/medium_6_11_12_21_7_23_55.jpeg)
