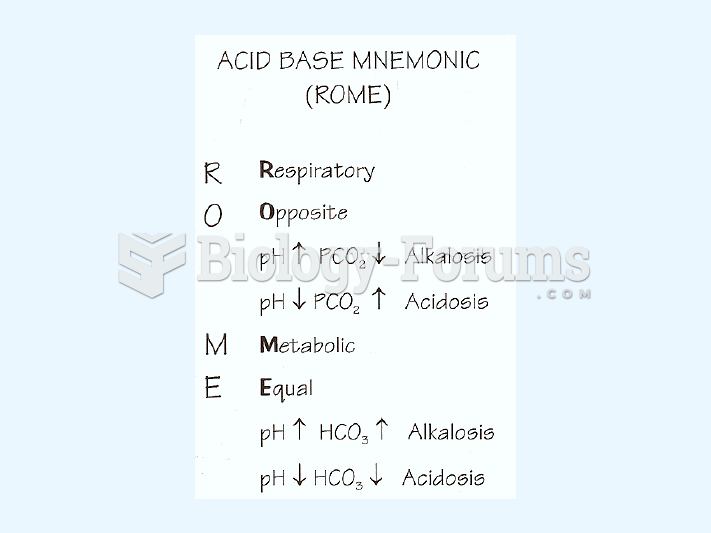
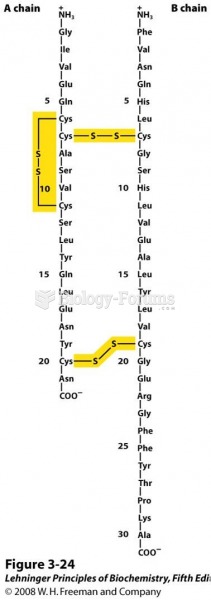
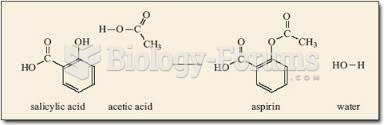
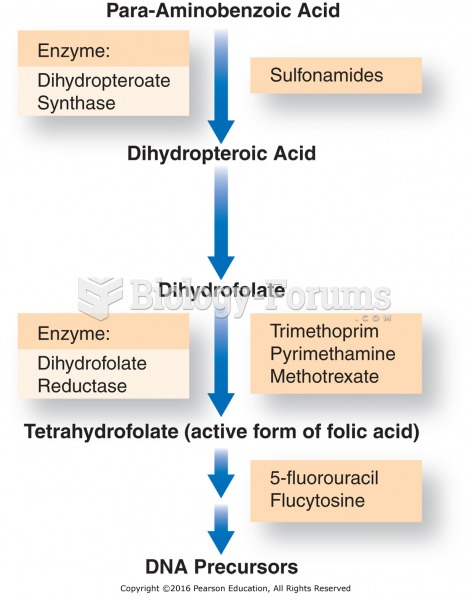
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.