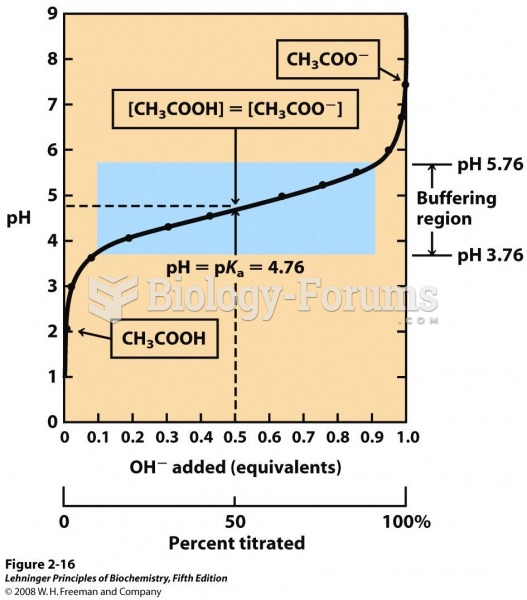
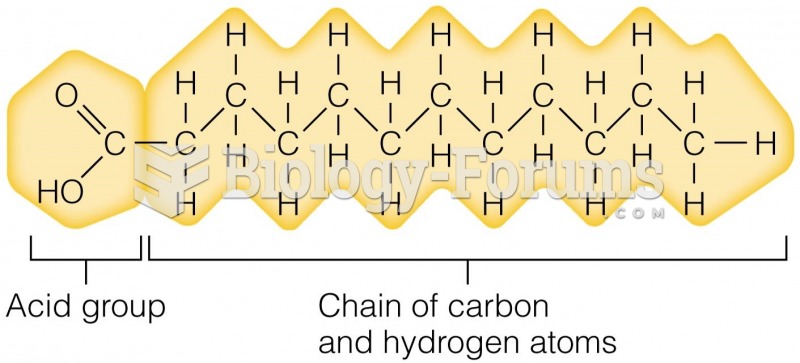
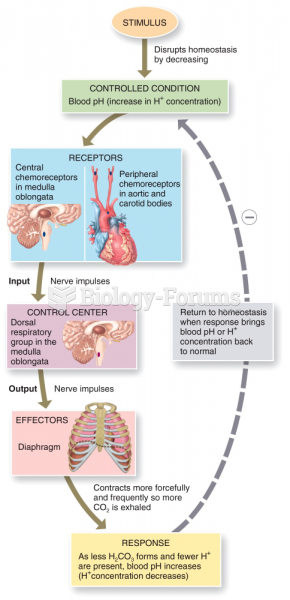
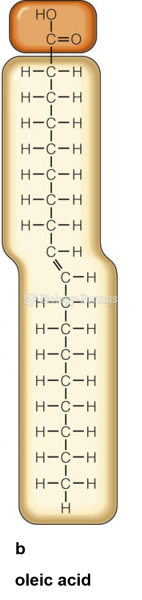
|
|
|
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
Acute bronchitis is an inflammation of the breathing tubes (bronchi), which causes increased mucus production and other changes. It is usually caused by bacteria or viruses, can be serious in people who have pulmonary or cardiac diseases, and can lead to pneumonia.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.