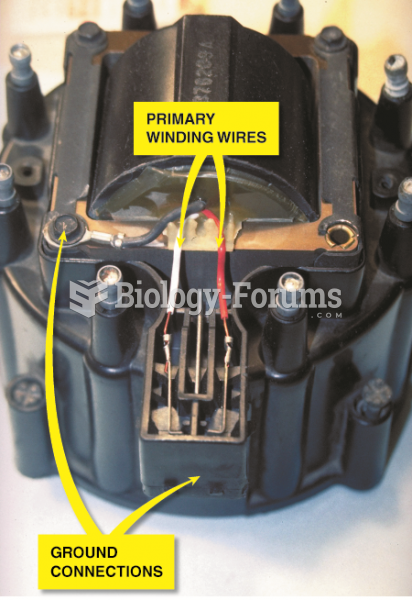
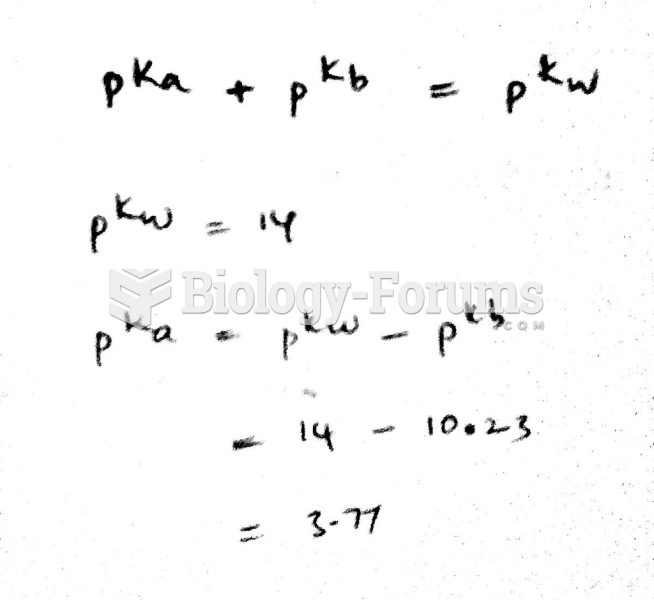|
|
|
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
There are immediate benefits of chiropractic adjustments that are visible via magnetic resonance imaging (MRI). It shows that spinal manipulation therapy is effective in decreasing pain and increasing the gaps between the vertebrae, reducing pressure that leads to pain.
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
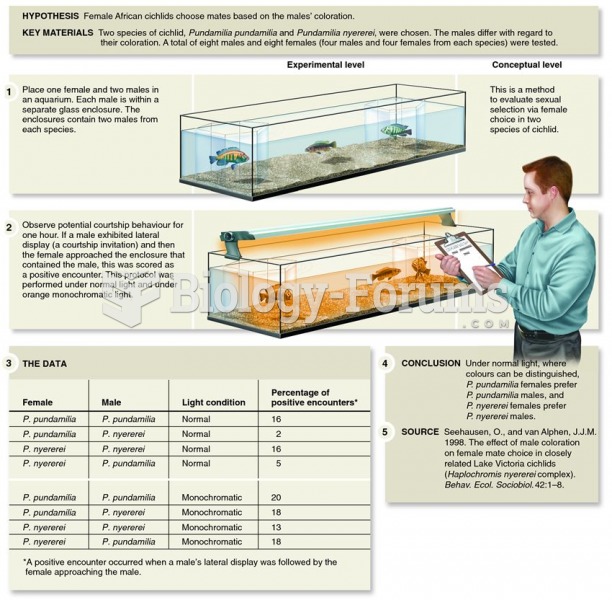 A study by Seehausen and van Alphen involving the effects of male coloration on female choice in Afr
A study by Seehausen and van Alphen involving the effects of male coloration on female choice in Afr
 To attain their goal of objectivity and accuracy in their research, sociologists must put away their ...
To attain their goal of objectivity and accuracy in their research, sociologists must put away their ...