This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
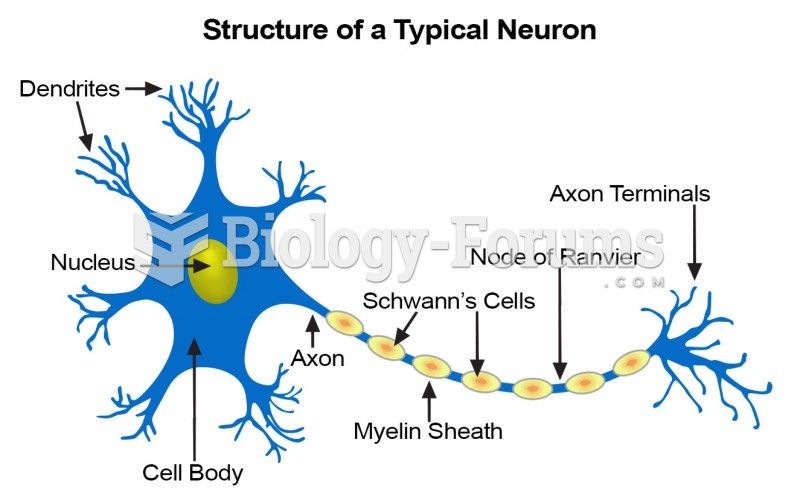
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.