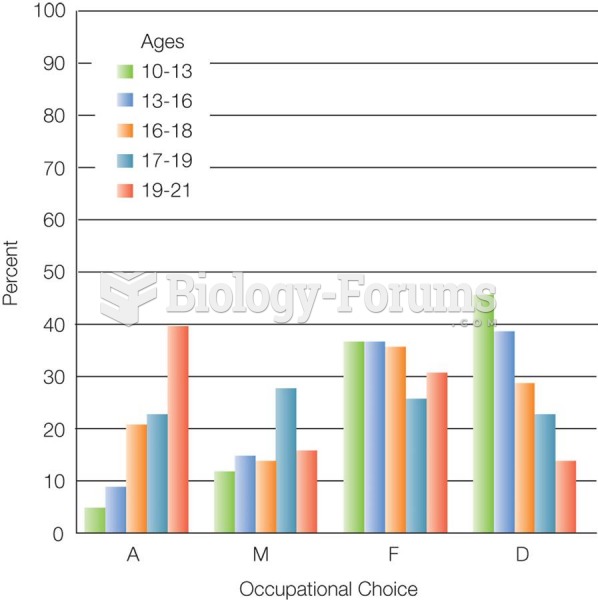
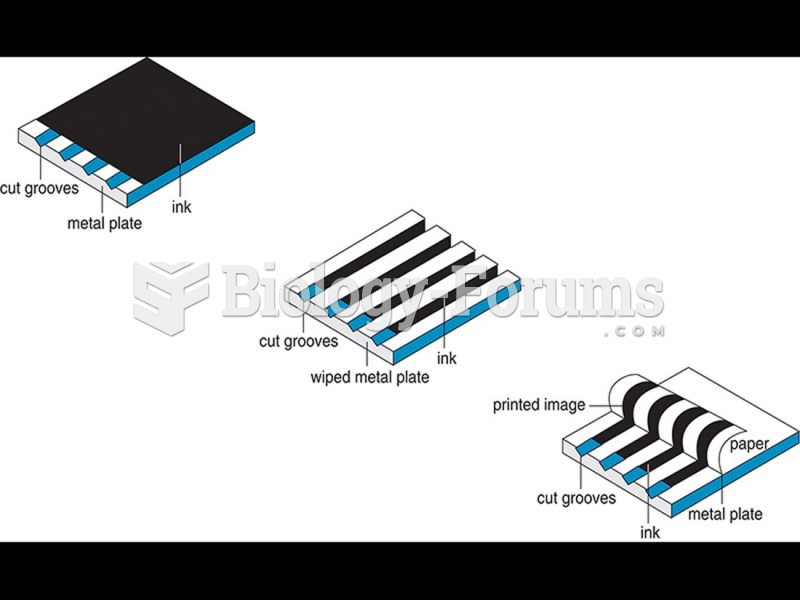
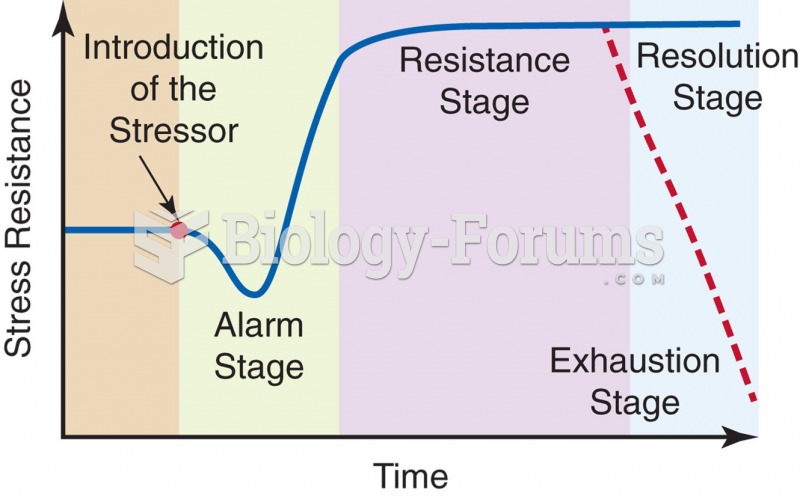
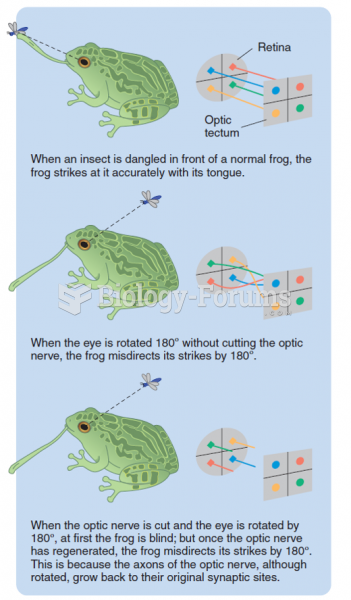
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
Although not all of the following muscle groups are commonly used, intramuscular injections may be given into the abdominals, biceps, calves, deltoids, gluteals, laterals, pectorals, quadriceps, trapezoids, and triceps.