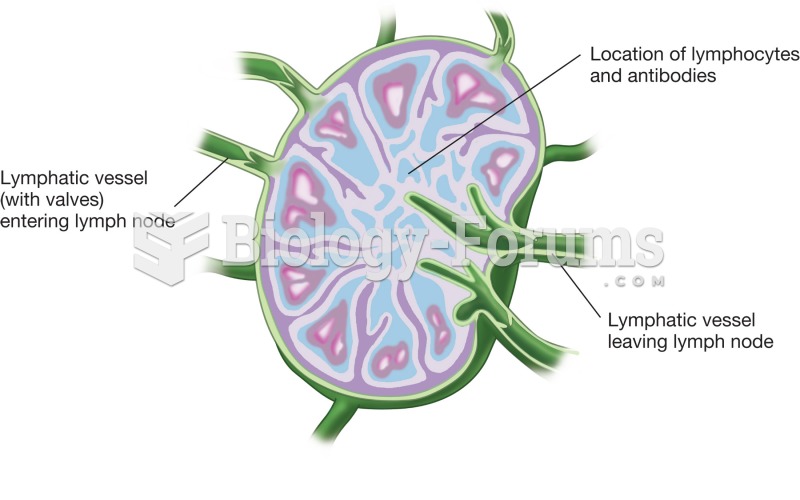
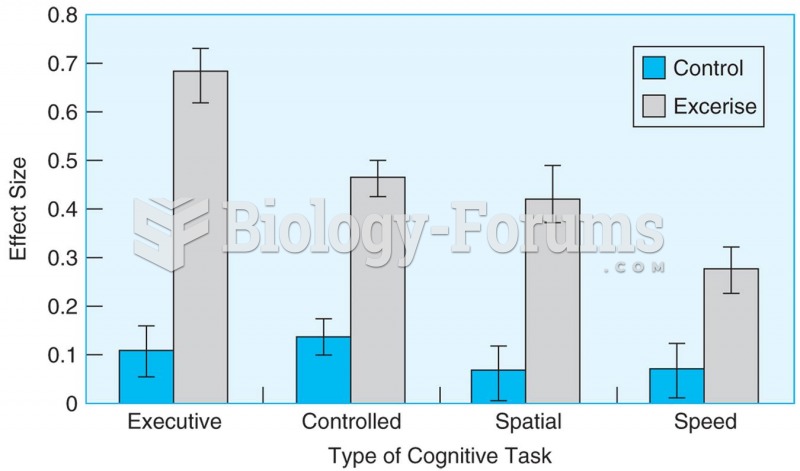
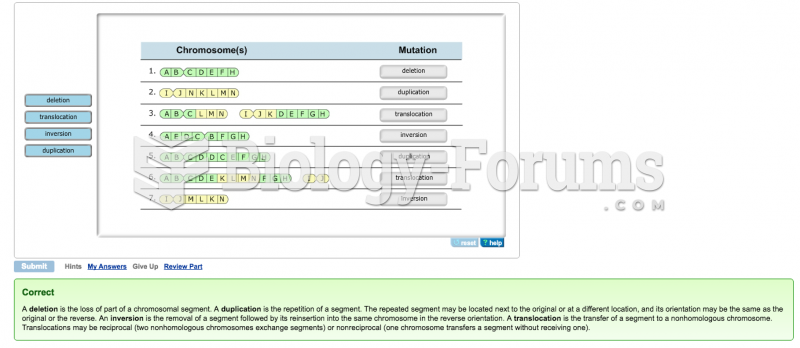
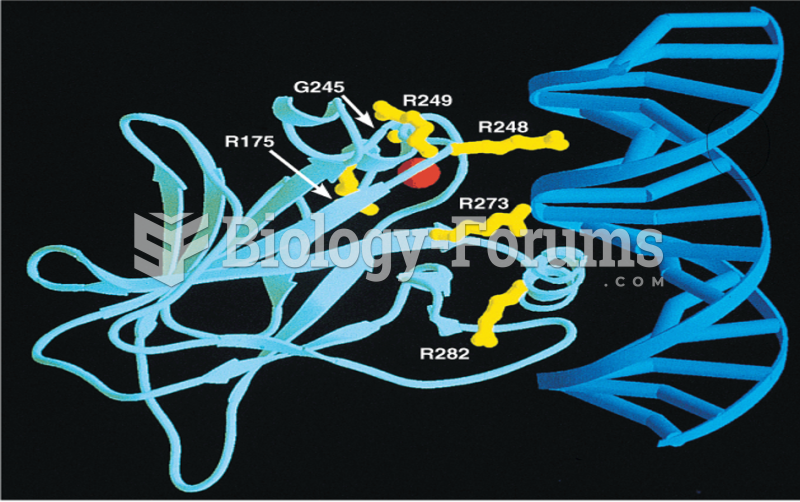
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.