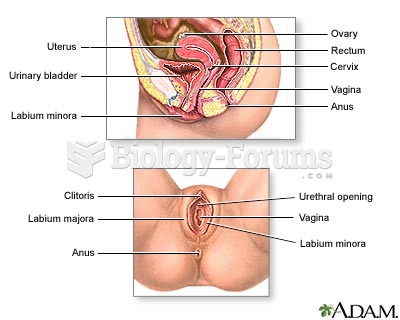
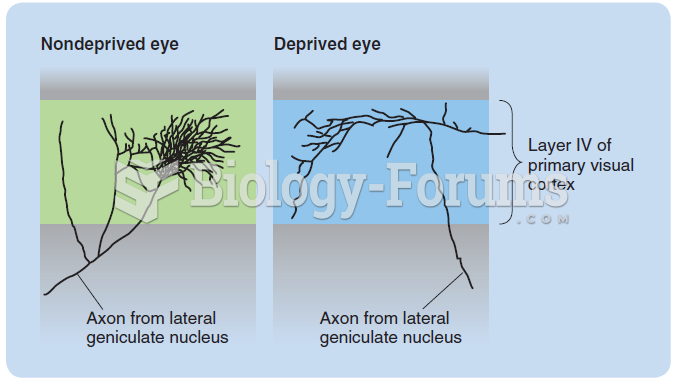
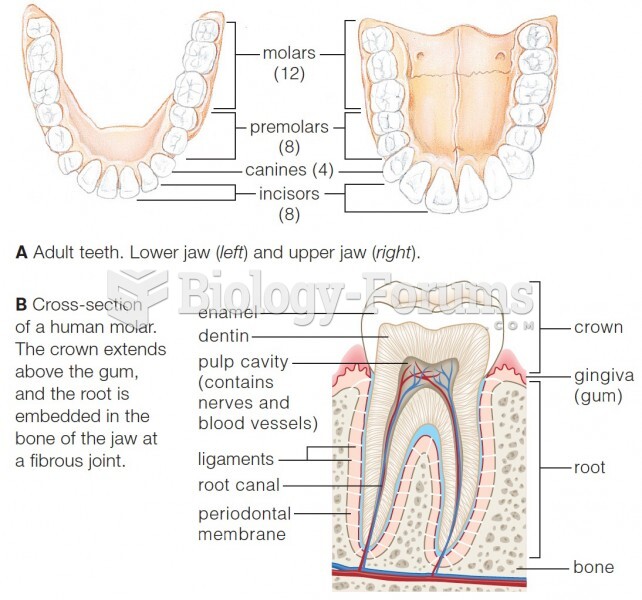
|
|
|
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Your skin wrinkles if you stay in the bathtub a long time because the outermost layer of skin (which consists of dead keratin) swells when it absorbs water. It is tightly attached to the skin below it, so it compensates for the increased area by wrinkling. This happens to the hands and feet because they have the thickest layer of dead keratin cells.
Illicit drug use costs the United States approximately $181 billion every year.
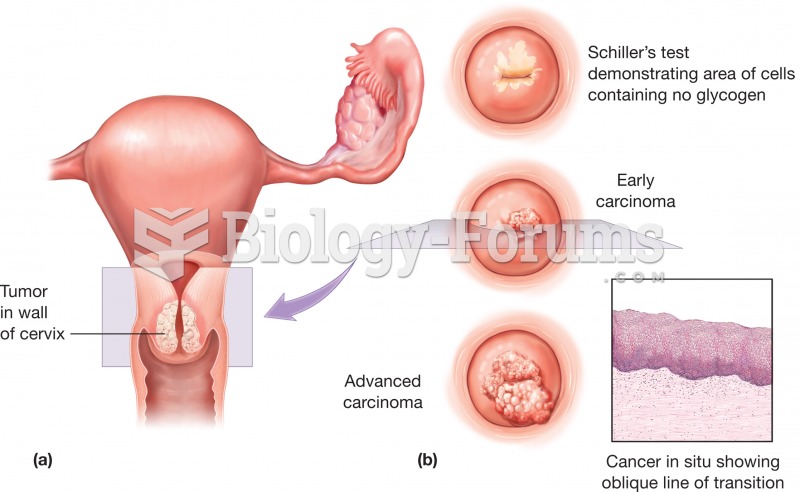 Cervical cancer (a) Top view of the uterus showing the presence of a tumor in the wall of the cervix
Cervical cancer (a) Top view of the uterus showing the presence of a tumor in the wall of the cervix
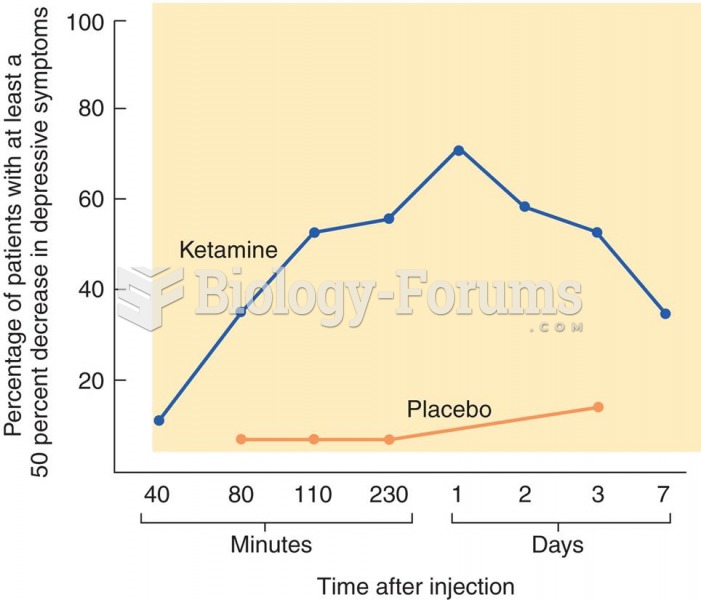 Treatment of Depression with Ketamine The graph shows the effects of ketamine on symptoms of depress
Treatment of Depression with Ketamine The graph shows the effects of ketamine on symptoms of depress