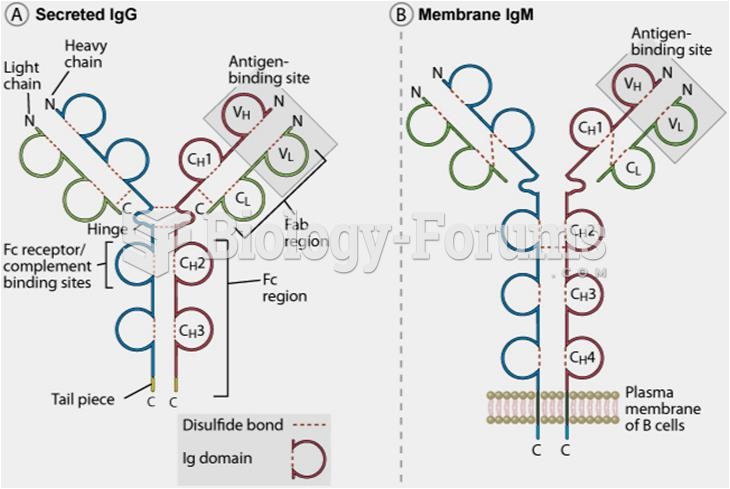
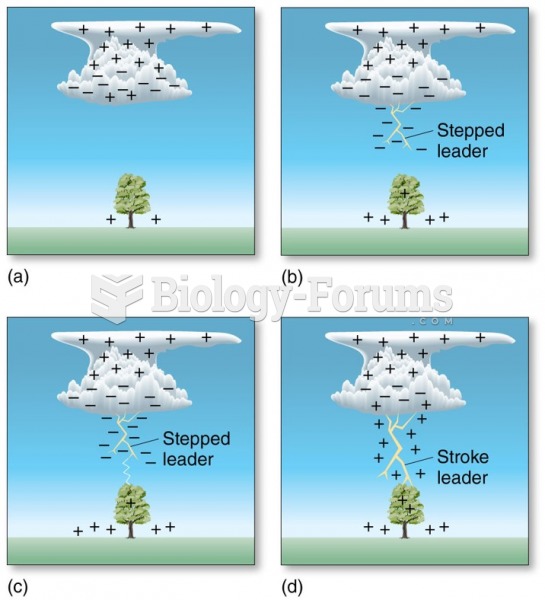
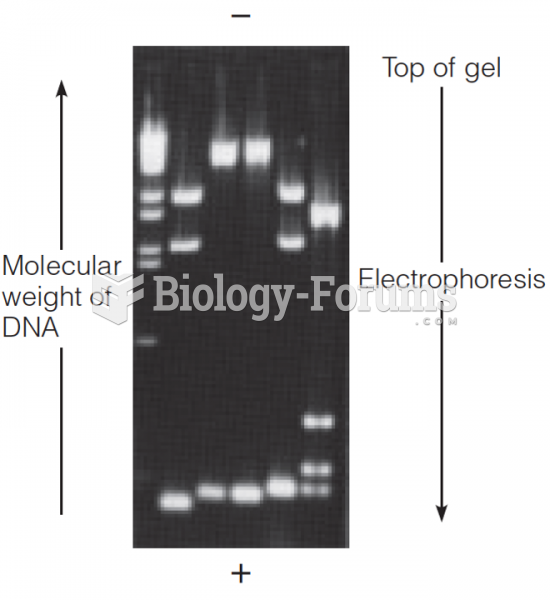
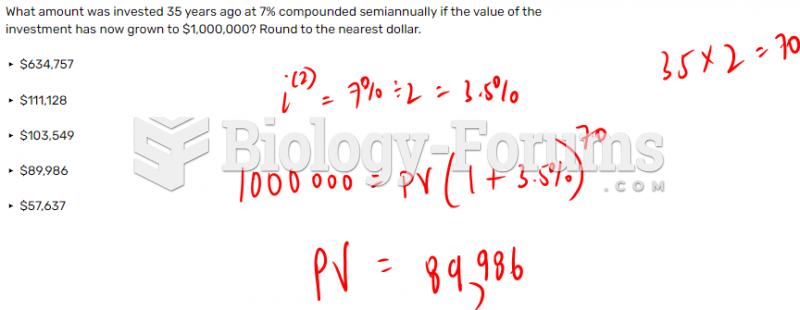
|
|
|
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
Medication errors are more common among seriously ill patients than with those with minor conditions.
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
The horizontal fraction bar was introduced by the Arabs.