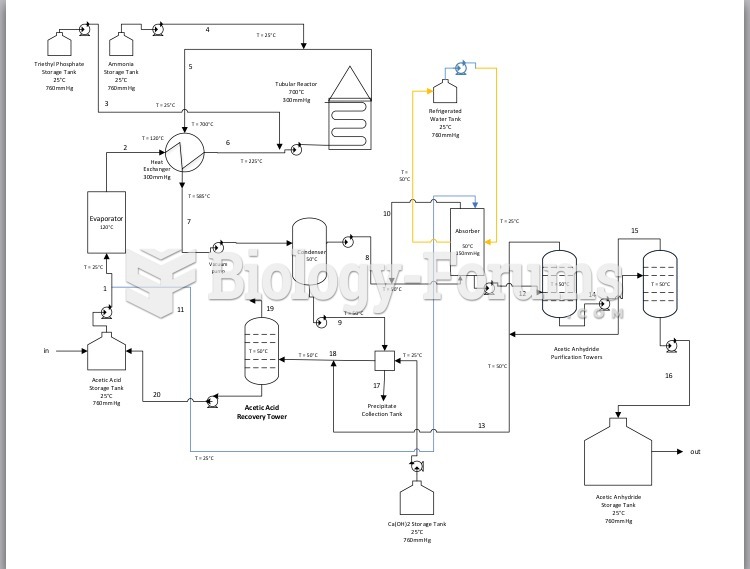
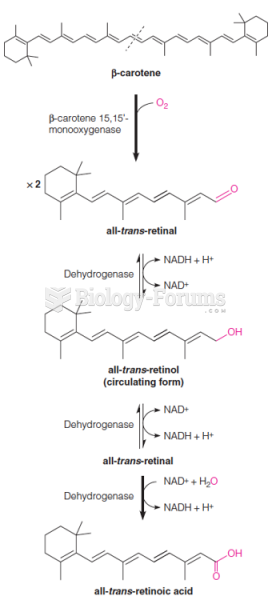
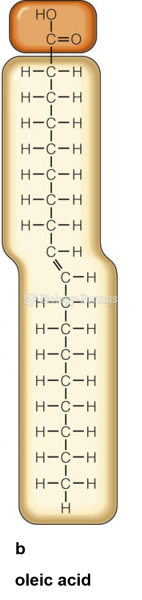
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Did you know?
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.
Did you know?
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
There are more bacteria in your mouth than there are people in the world.