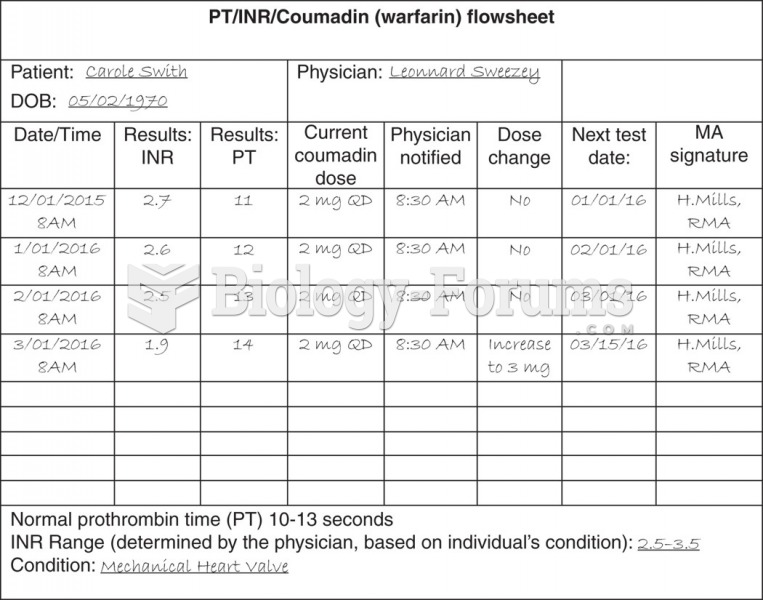
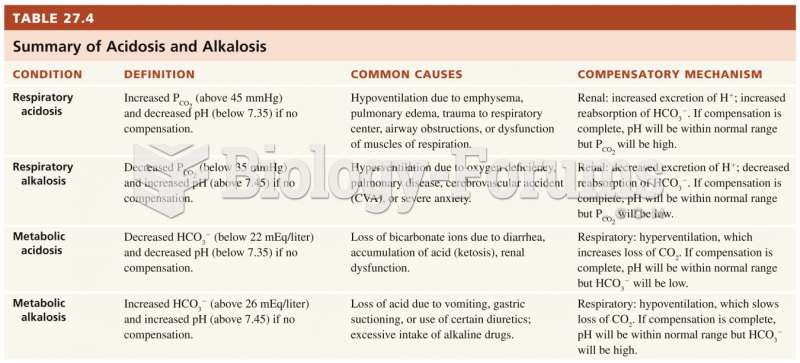
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.