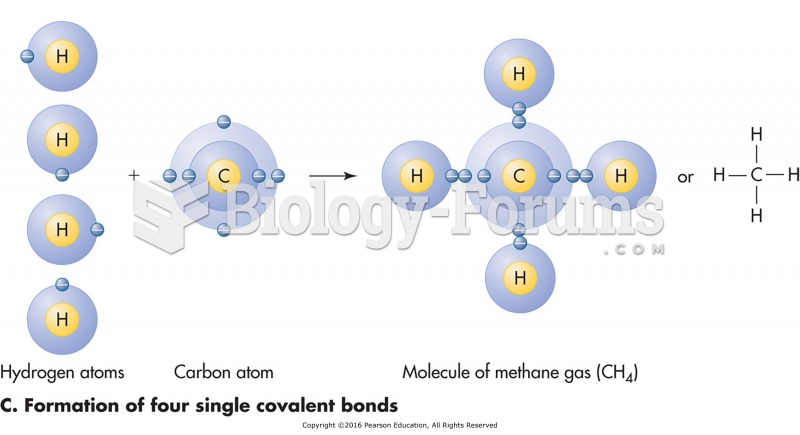
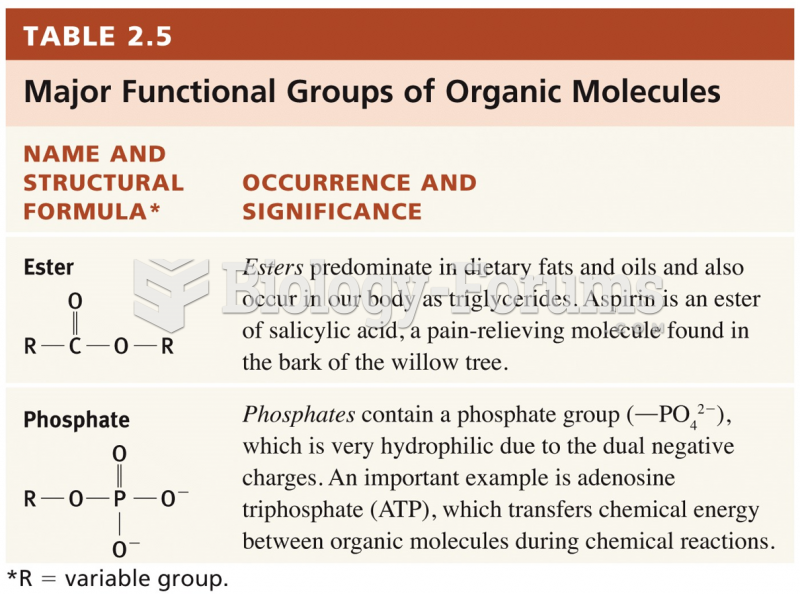
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Did you know?
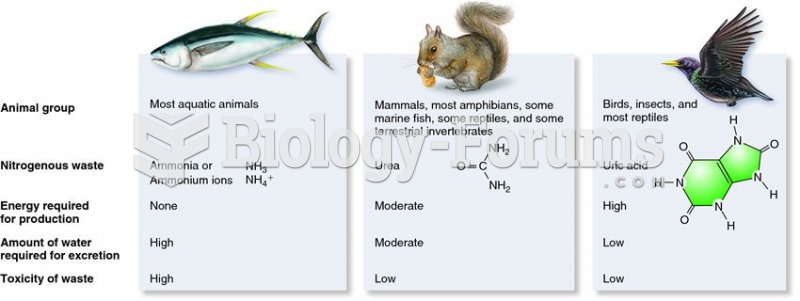
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.