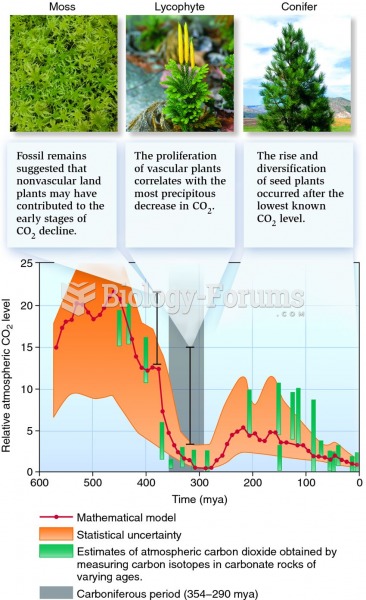
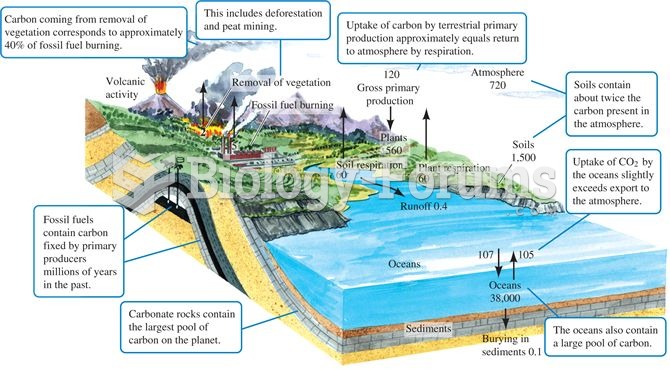
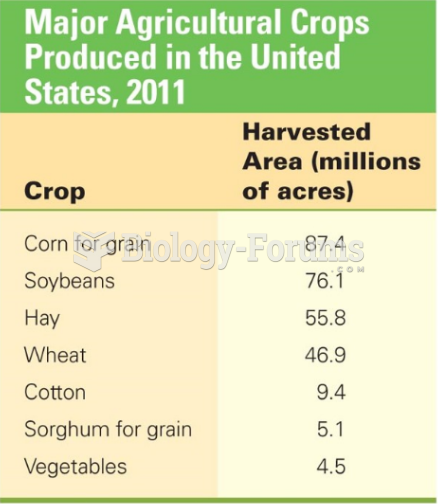
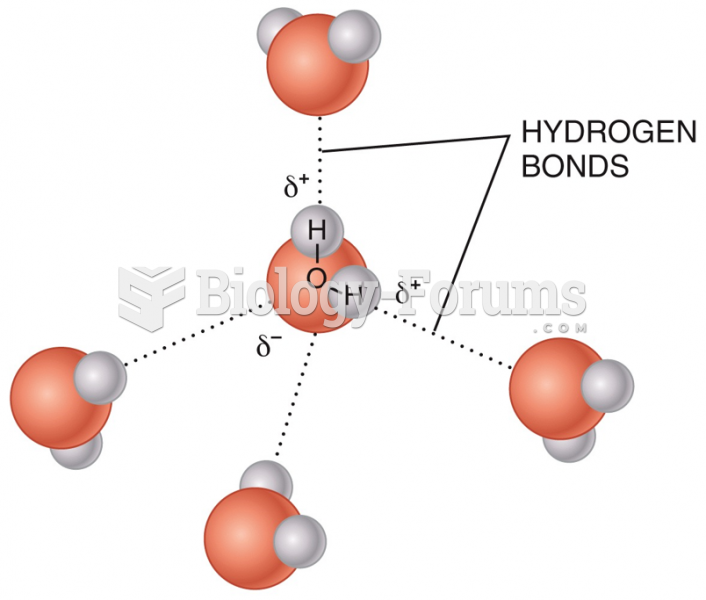
|
|
|
Excessive alcohol use costs the country approximately $235 billion every year.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.