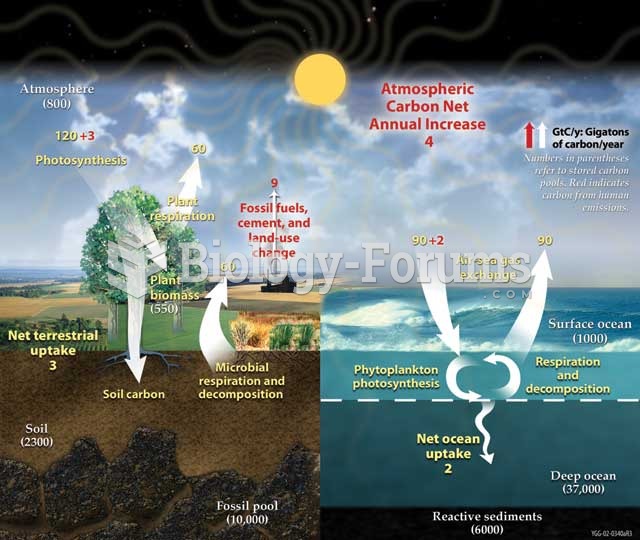
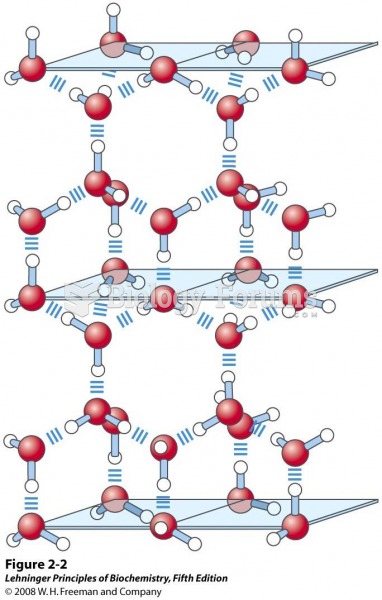
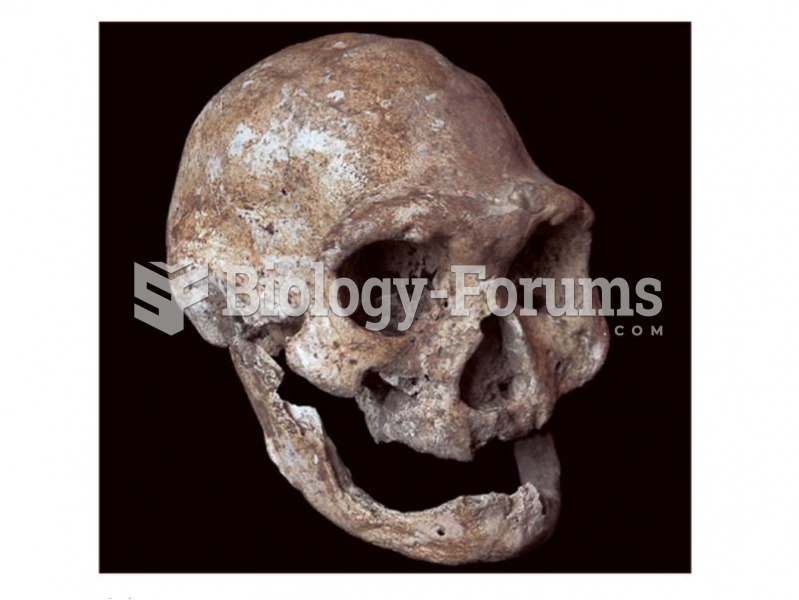
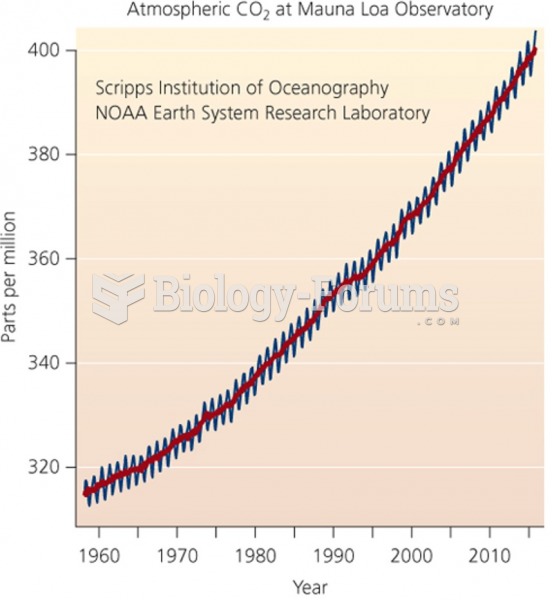
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Did you know?
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.