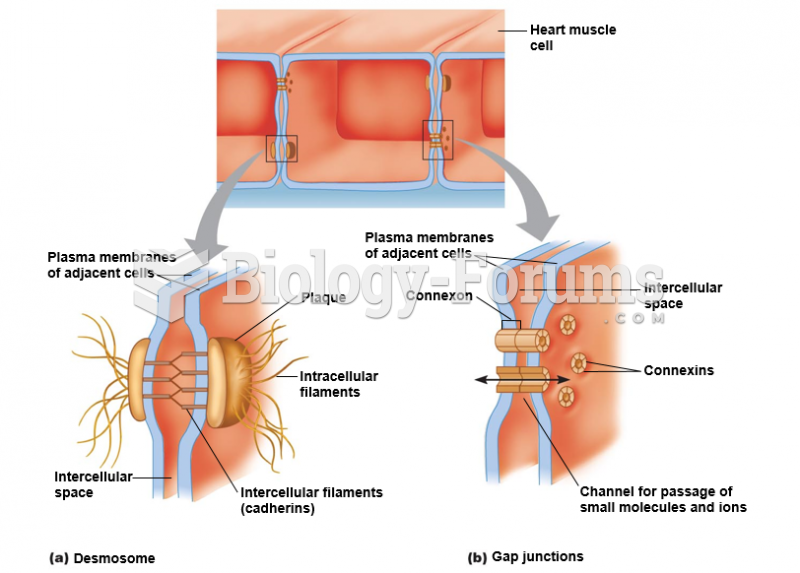
|
|
|
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Acute bronchitis is an inflammation of the breathing tubes (bronchi), which causes increased mucus production and other changes. It is usually caused by bacteria or viruses, can be serious in people who have pulmonary or cardiac diseases, and can lead to pneumonia.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
 One danger of a wire fence is that, as shown in this photo, it is practically invisible; a running a
One danger of a wire fence is that, as shown in this photo, it is practically invisible; a running a
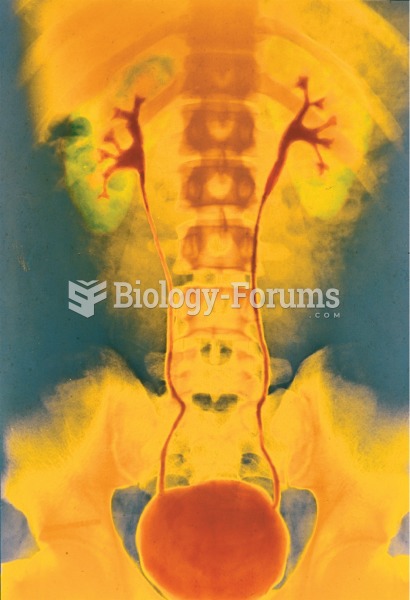 Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra