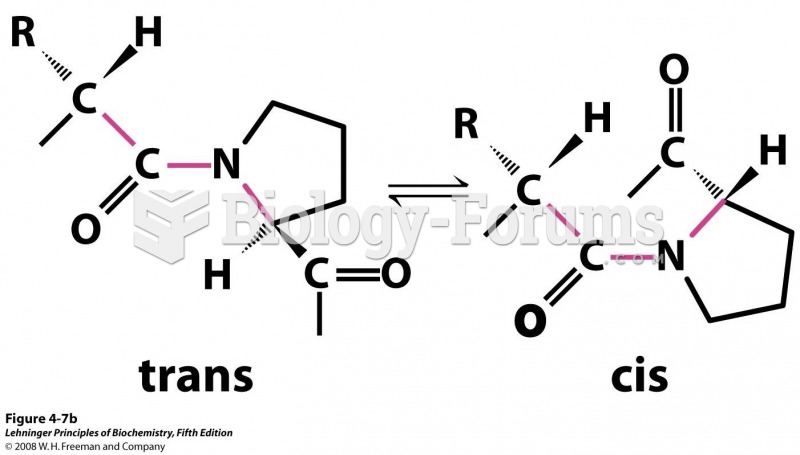
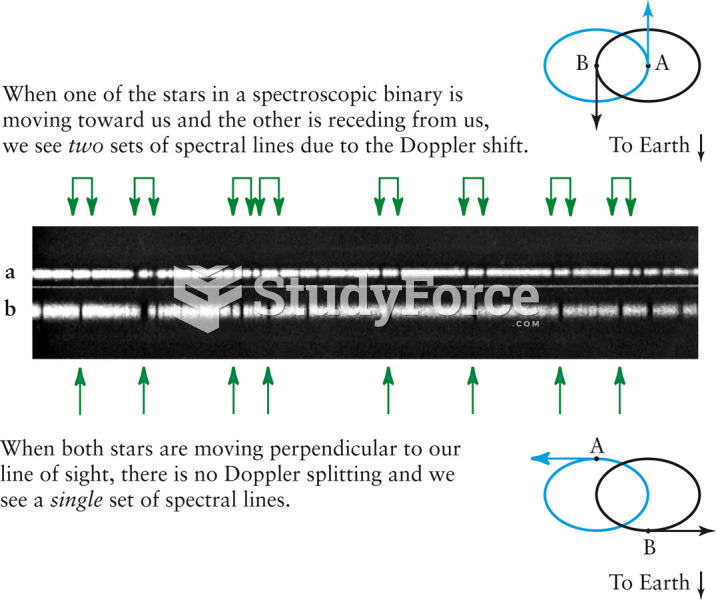
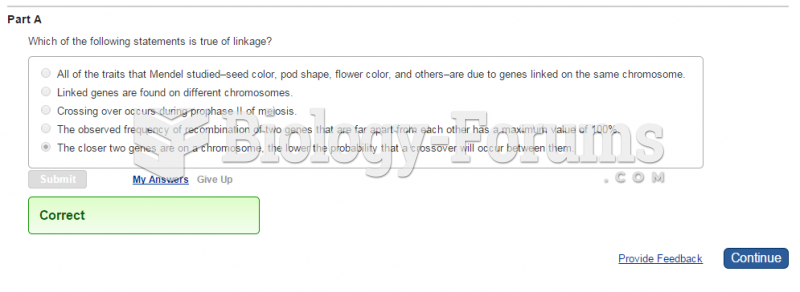
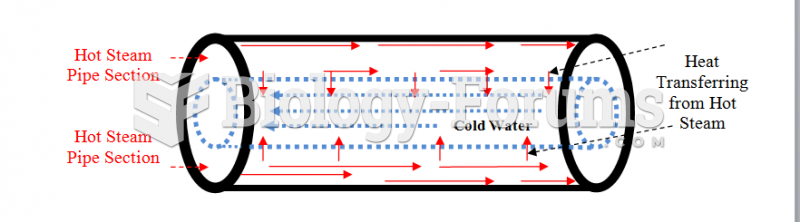
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.