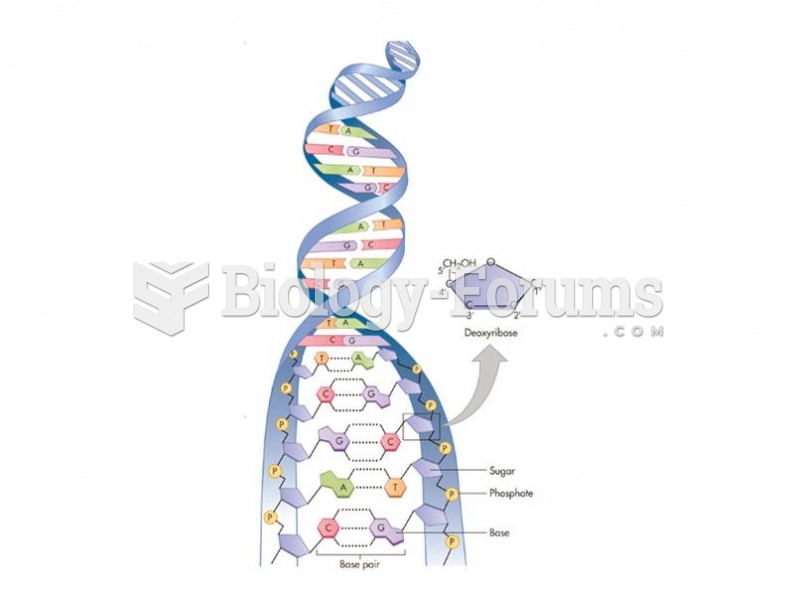
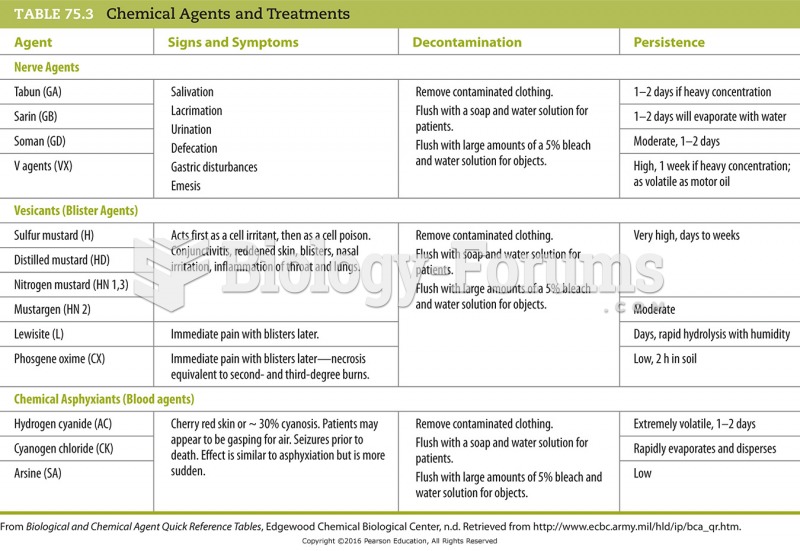
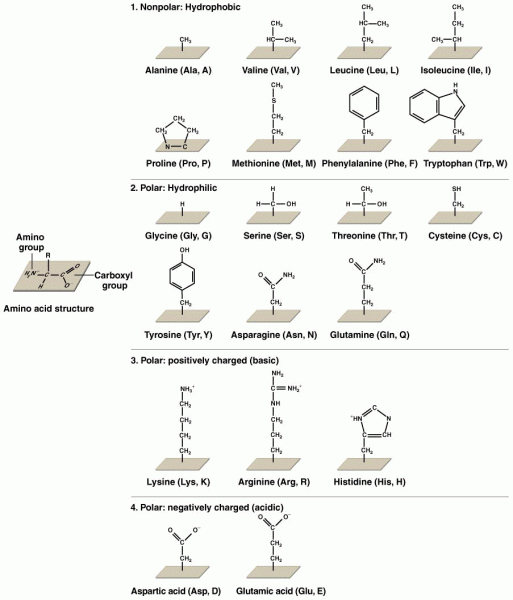
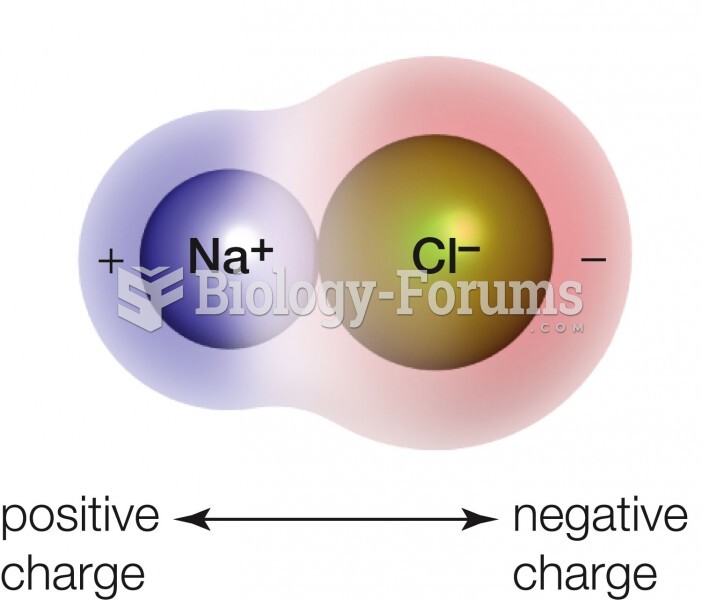
|
|
|
In 1864, the first barbiturate (barbituric acid) was synthesized.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
It is believed that the Incas used anesthesia. Evidence supports the theory that shamans chewed cocoa leaves and drilled holes into the heads of patients (letting evil spirits escape), spitting into the wounds they made. The mixture of cocaine, saliva, and resin numbed the site enough to allow hours of drilling.
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.