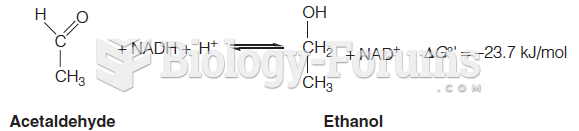
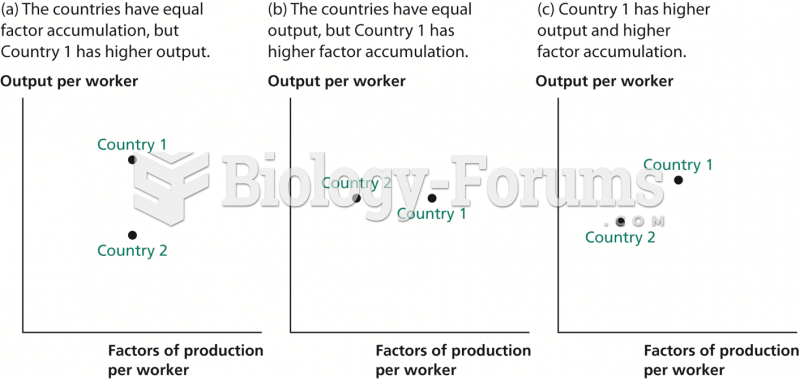
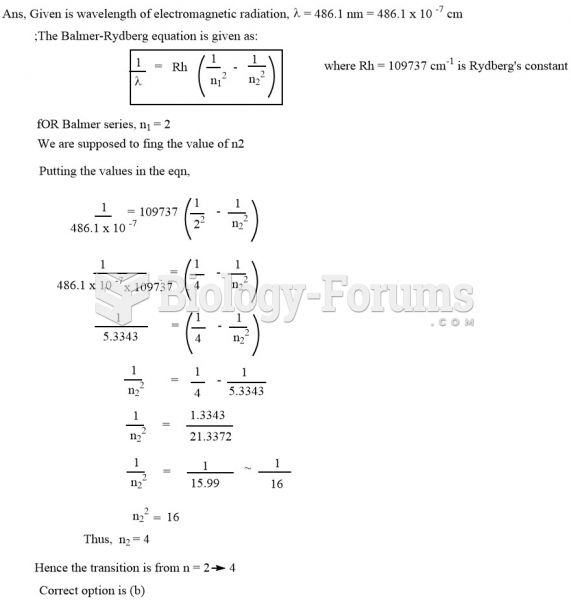
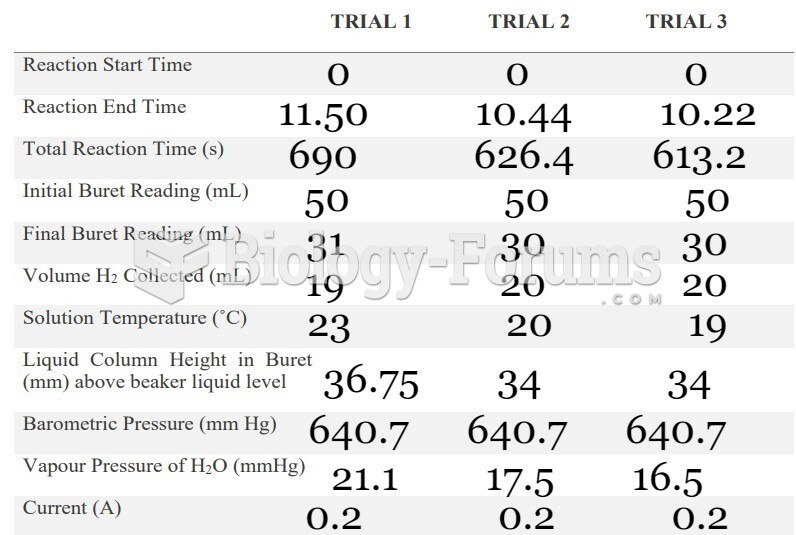
|
|
|
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.