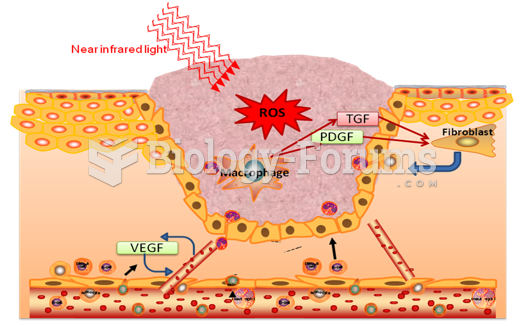
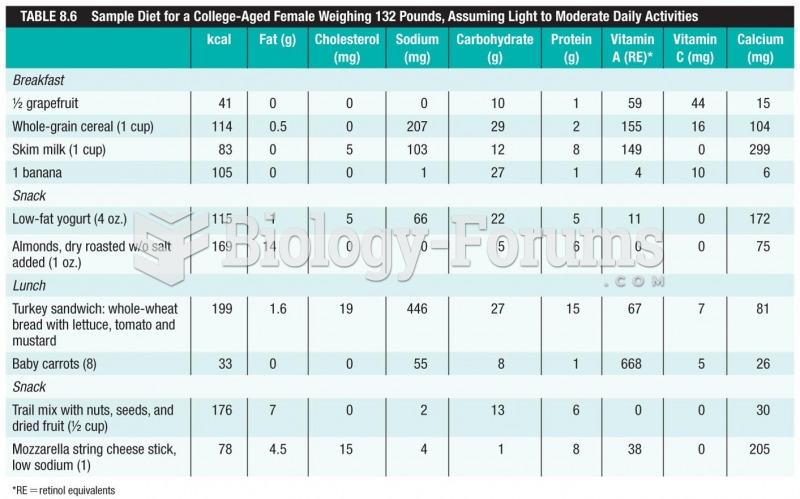
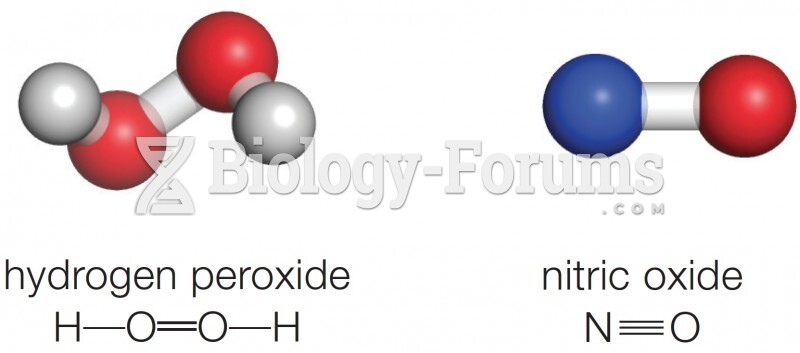
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
Did you know?
The FDA recognizes 118 routes of administration.