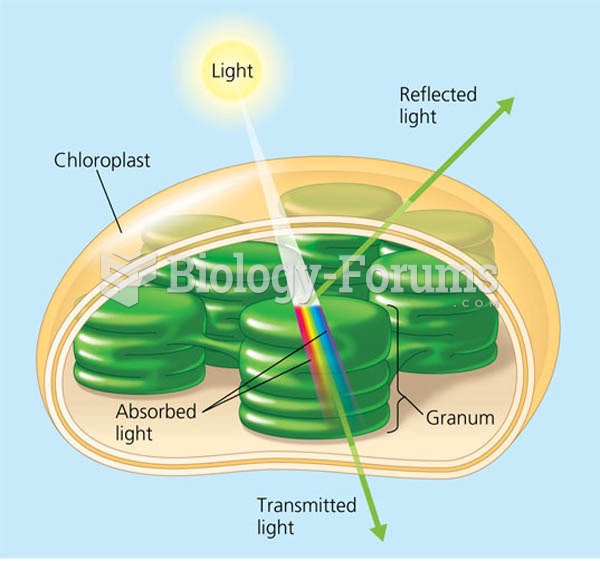
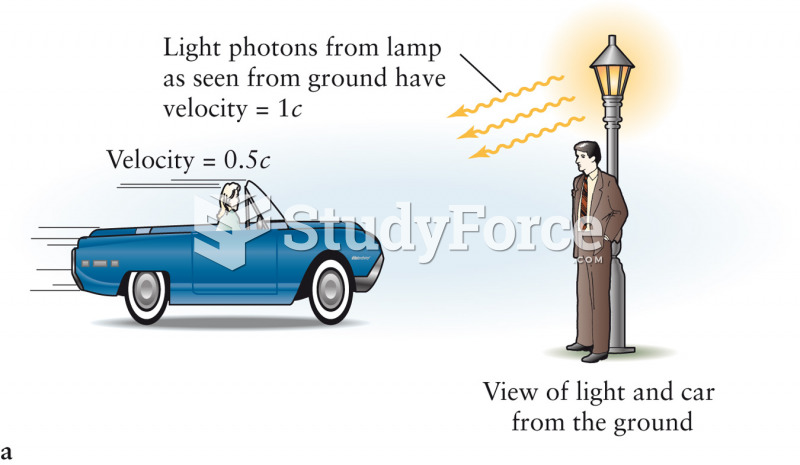
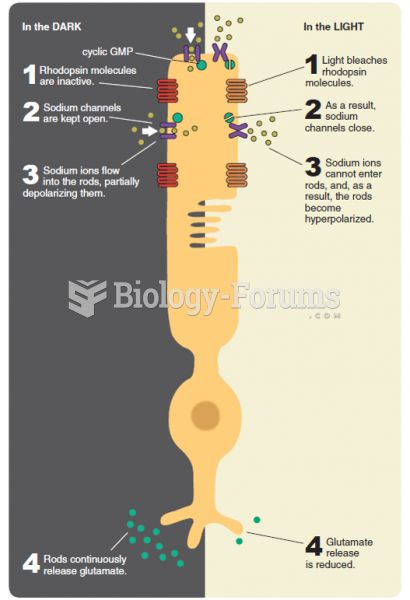
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.