This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
Did you know?
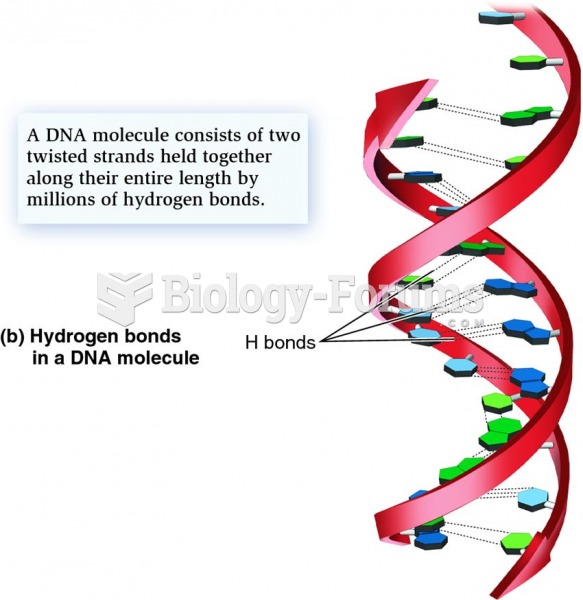
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.