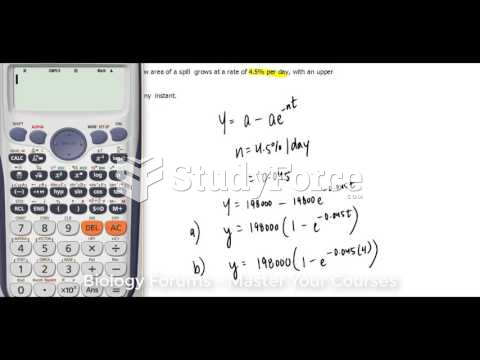
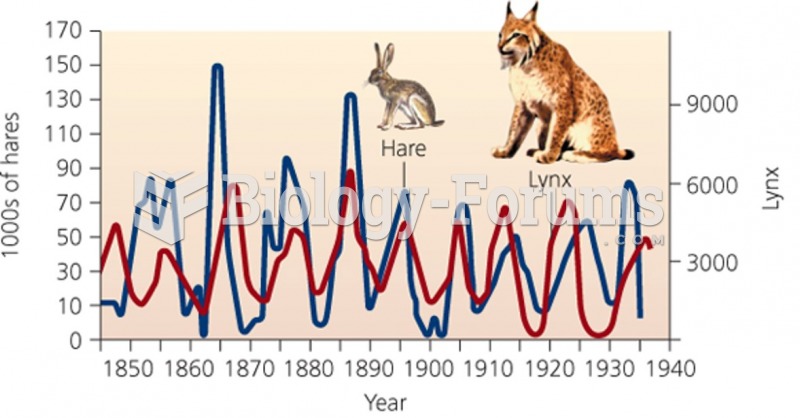
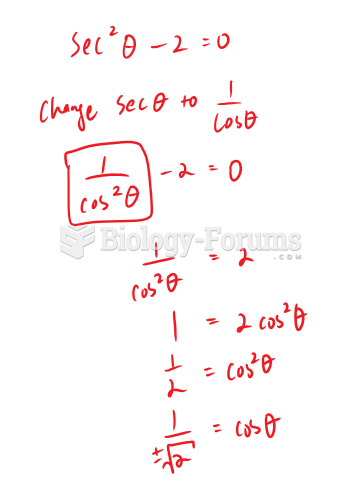
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.
Did you know?
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Did you know?
The longest a person has survived after a heart transplant is 24 years.