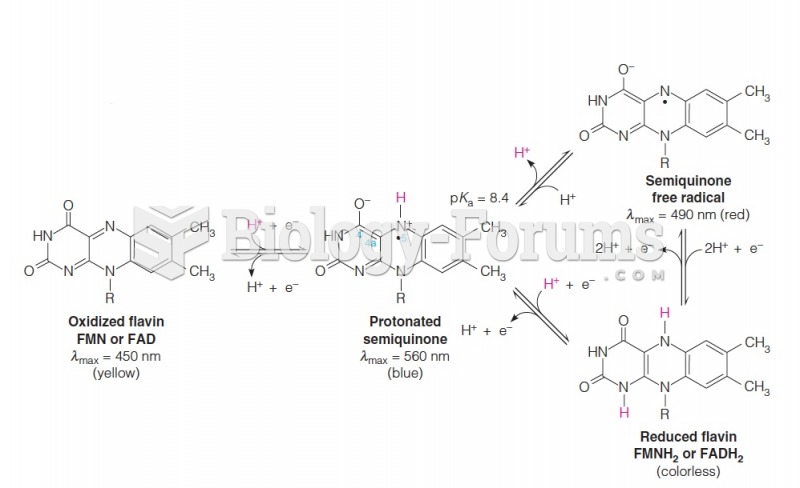
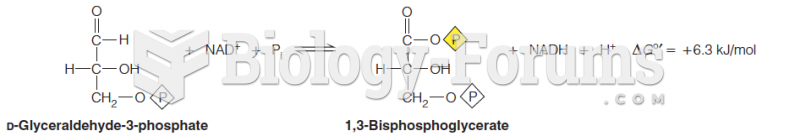
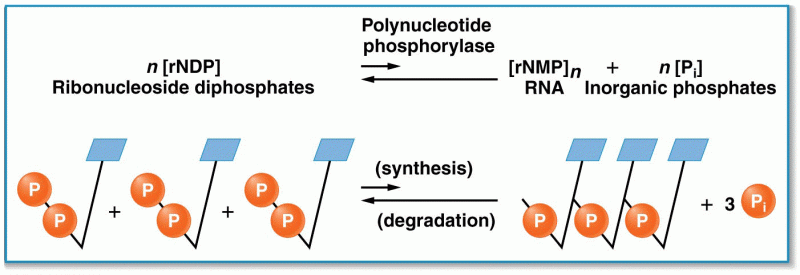
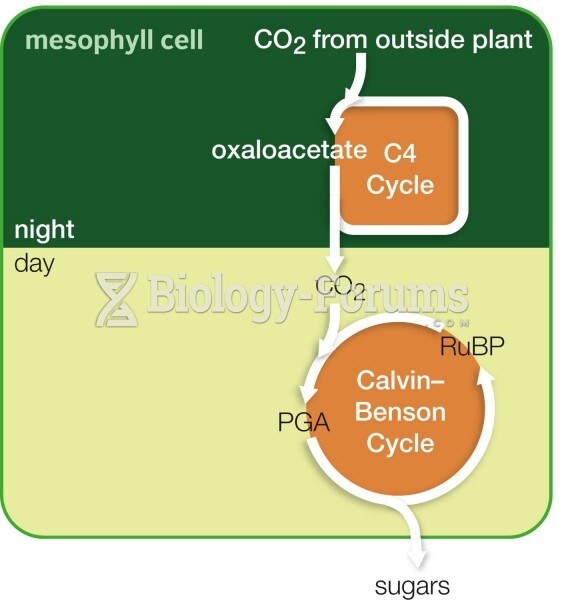
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.