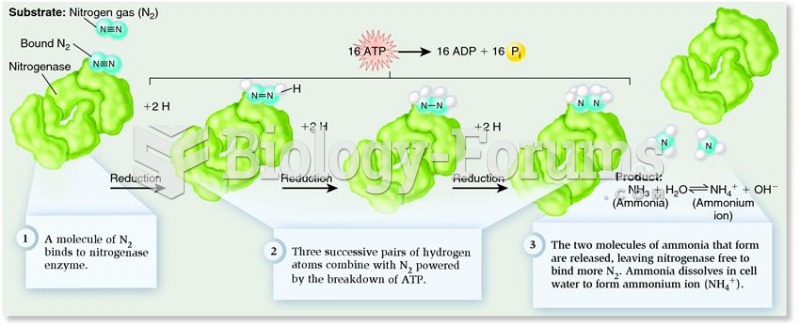
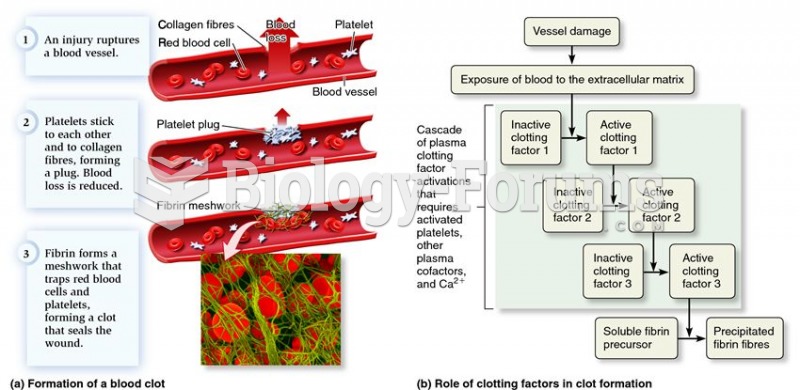
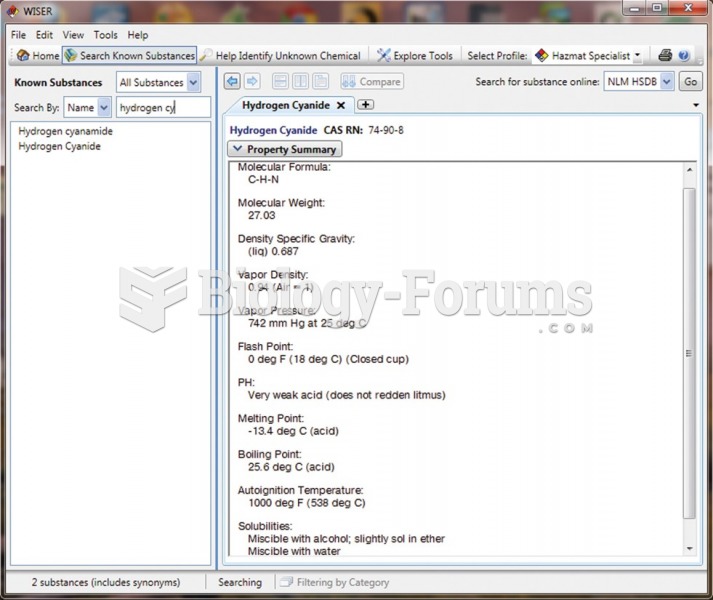
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.