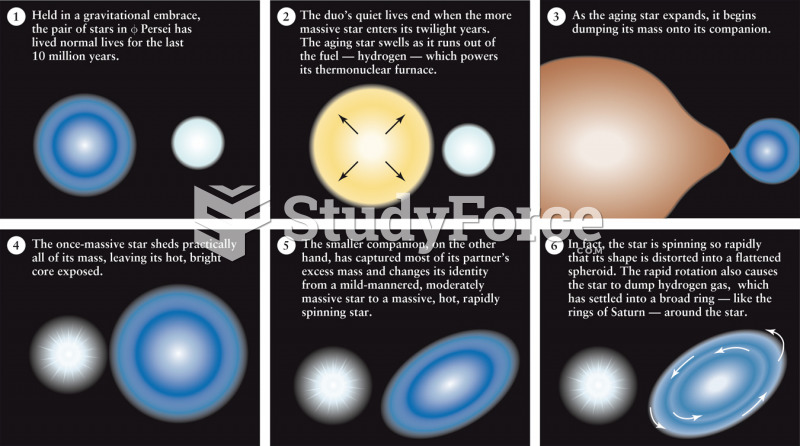
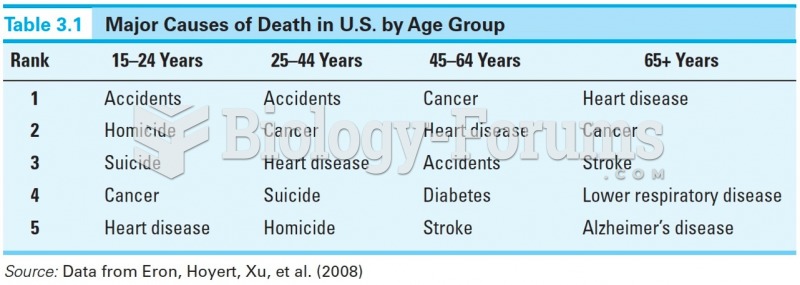
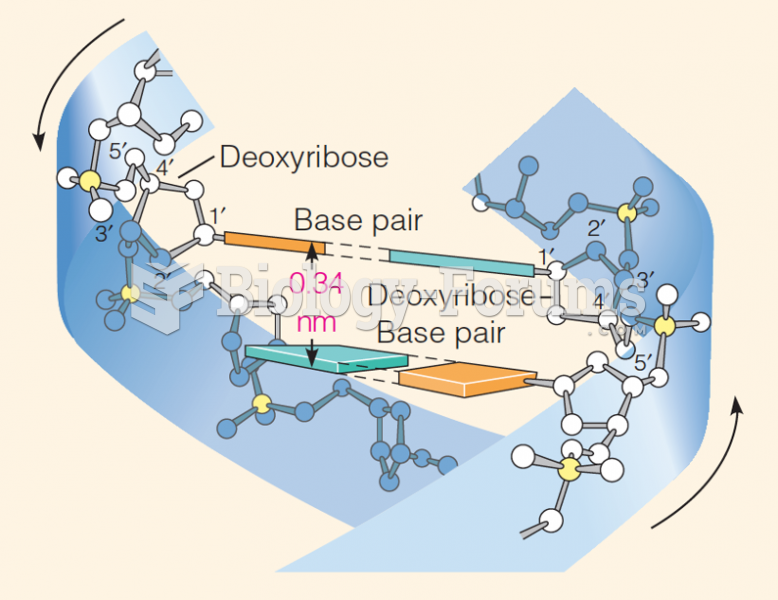
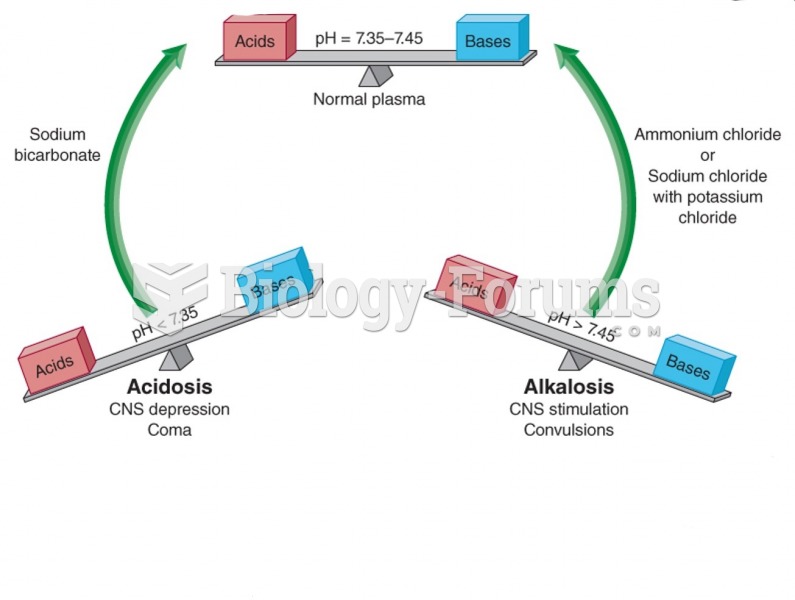
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.