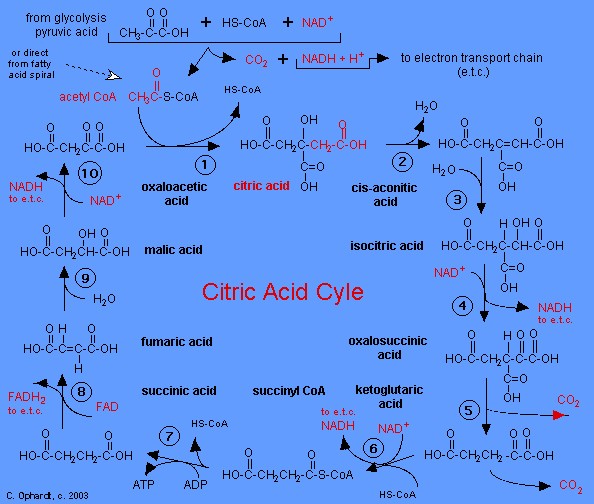
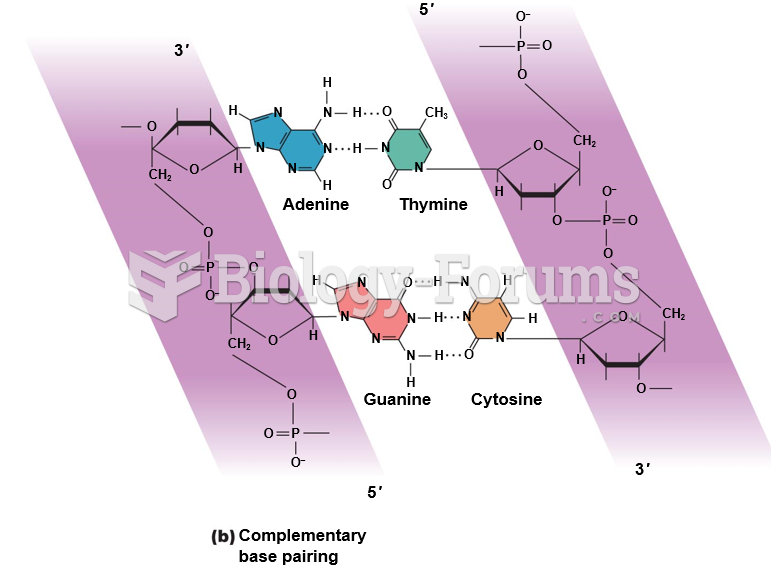
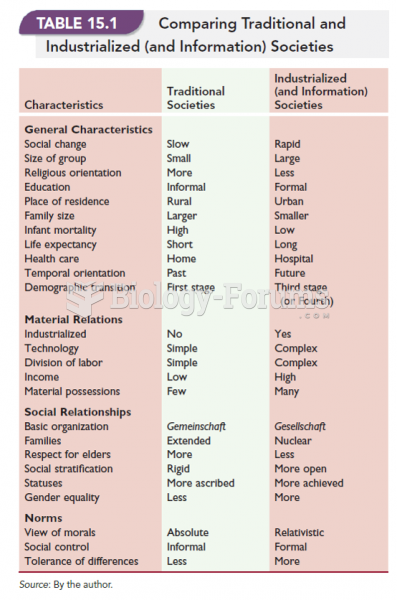
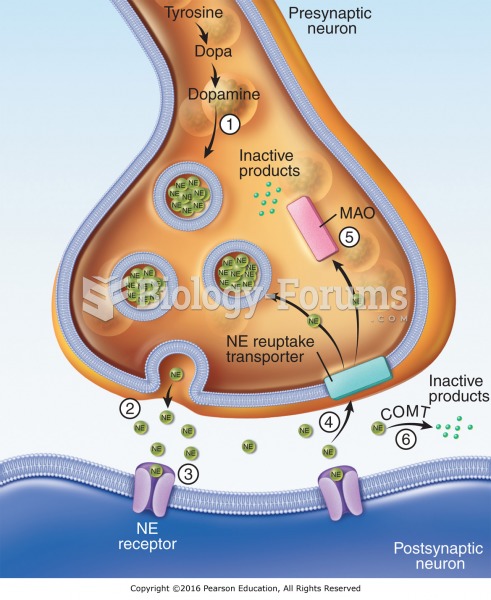
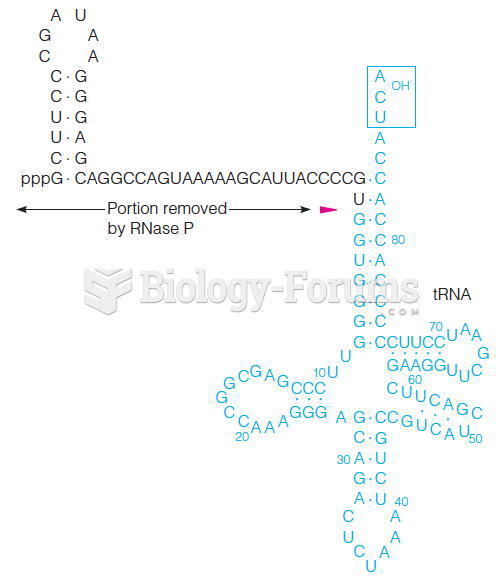
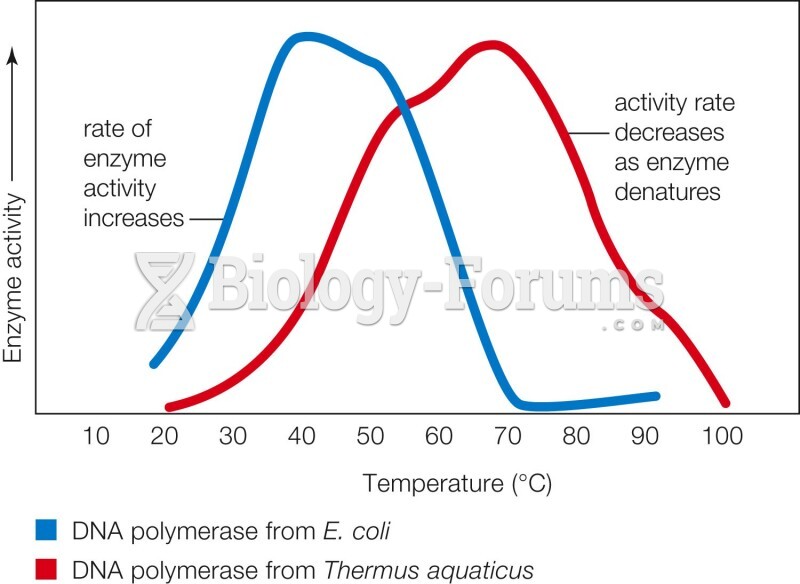
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.