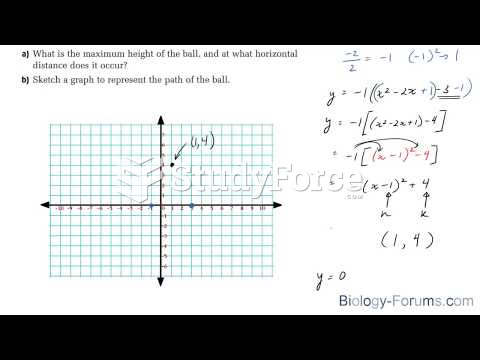
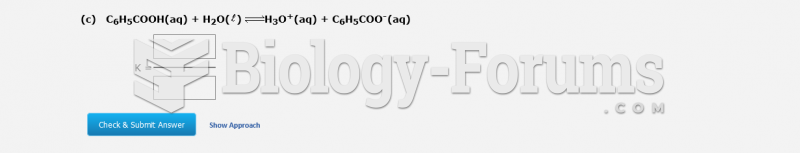
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Did you know?
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.