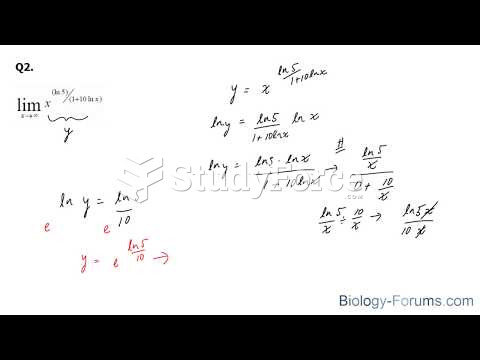
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.