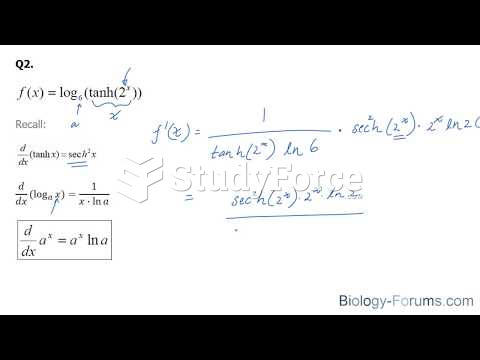
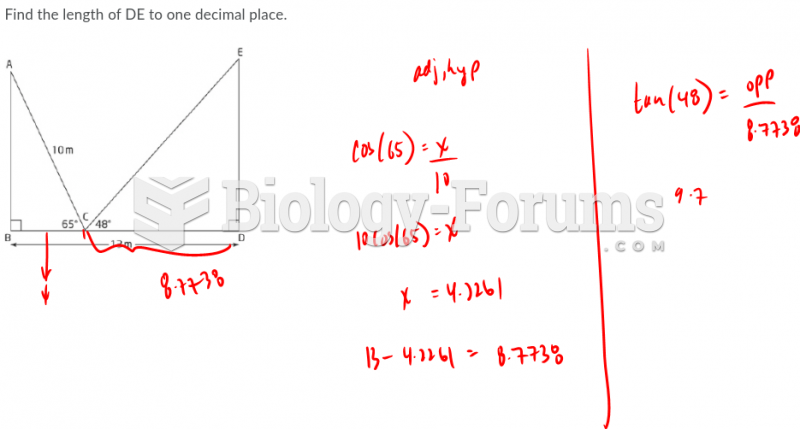
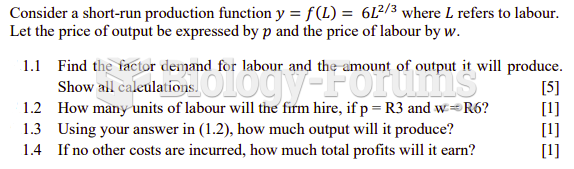
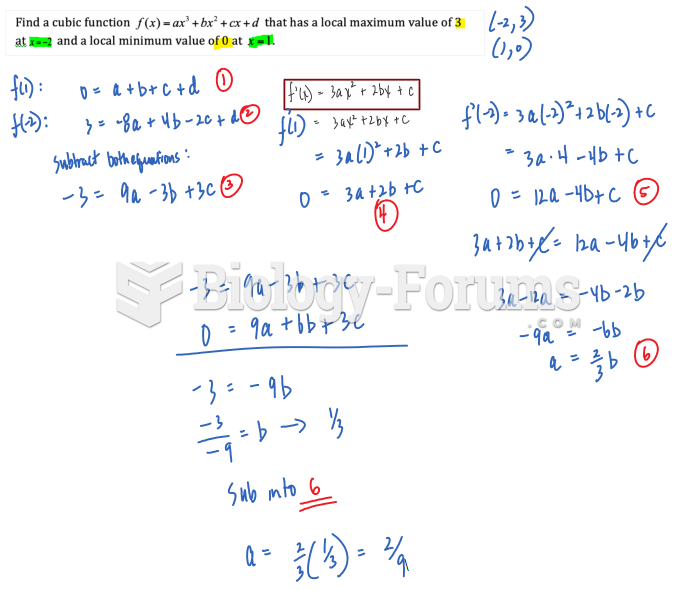
|
|
|
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.