|
|
|
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
 Each of the eight injectors shown are producing a correct spray pattern for the applications. While ...
Each of the eight injectors shown are producing a correct spray pattern for the applications. While ...
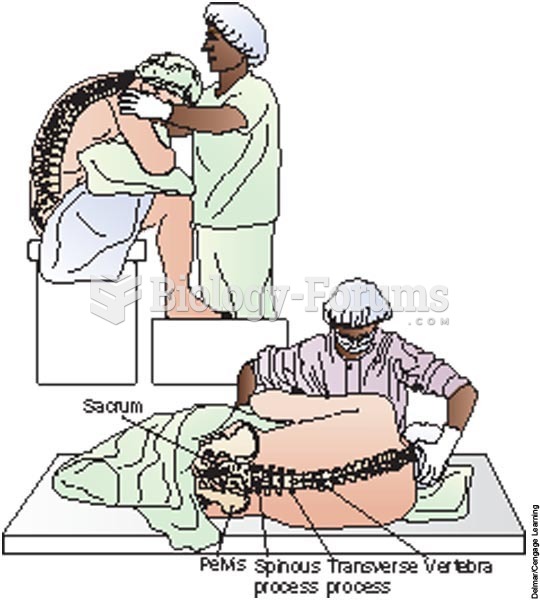
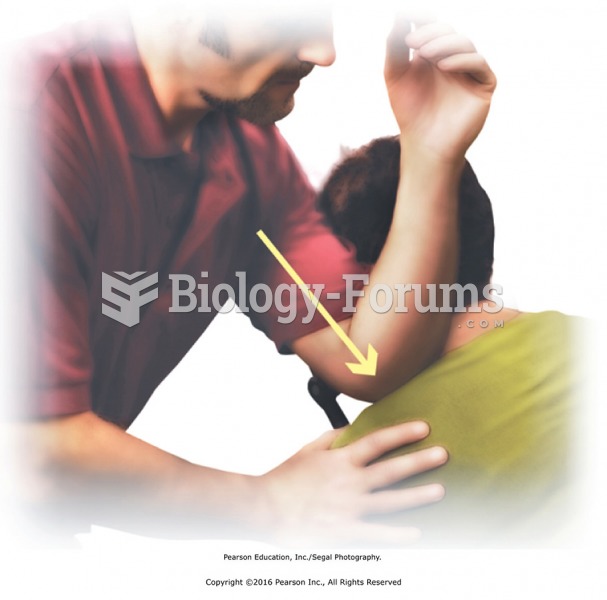
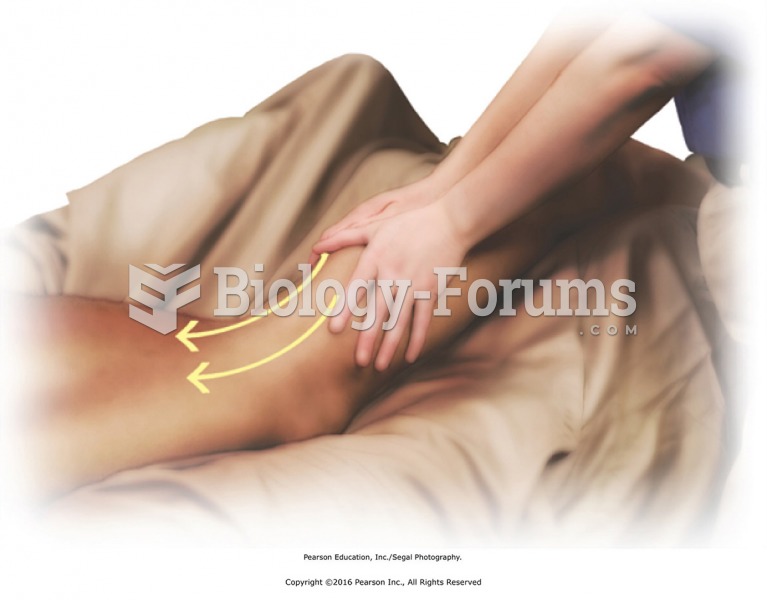 Stand at the side of the table facing the head. Apply lubricant with effleurage from ankle to hip ...
Stand at the side of the table facing the head. Apply lubricant with effleurage from ankle to hip ...