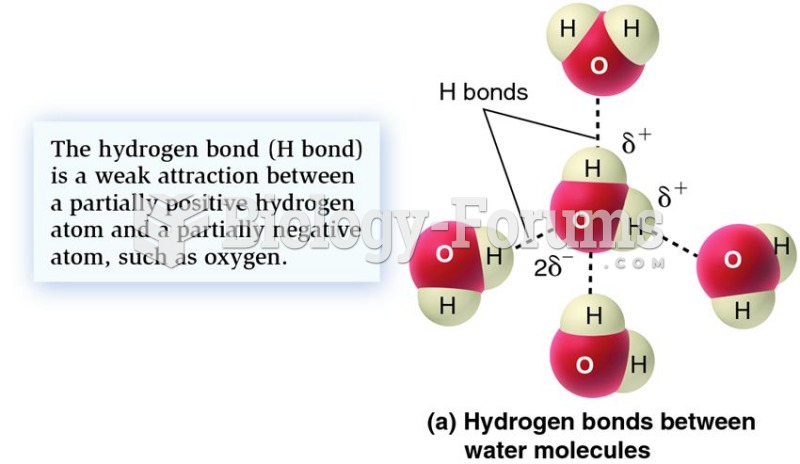
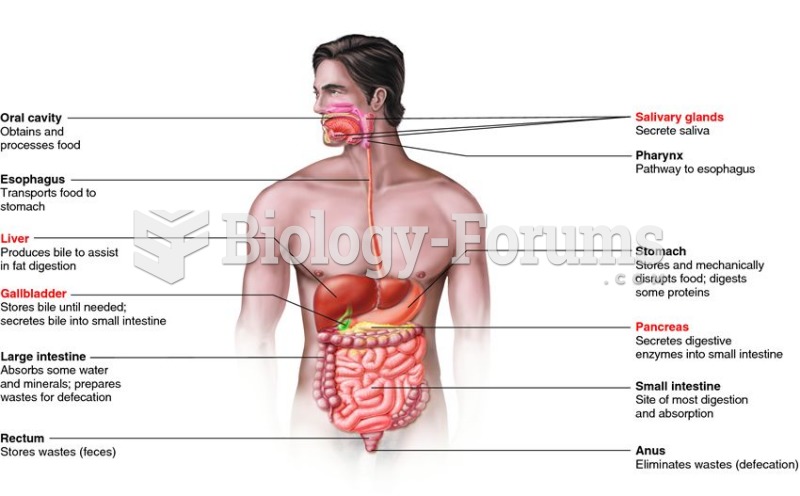
|
|
|
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
On average, the stomach produces 2 L of hydrochloric acid per day.
Fatal fungal infections may be able to resist newer antifungal drugs. Globally, fungal infections are often fatal due to the lack of access to multiple antifungals, which may be required to be utilized in combination. Single antifungals may not be enough to stop a fungal infection from causing the death of a patient.
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.