|
|
|
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Asthma cases in Americans are about 75% higher today than they were in 1980.
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
 As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...
As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...
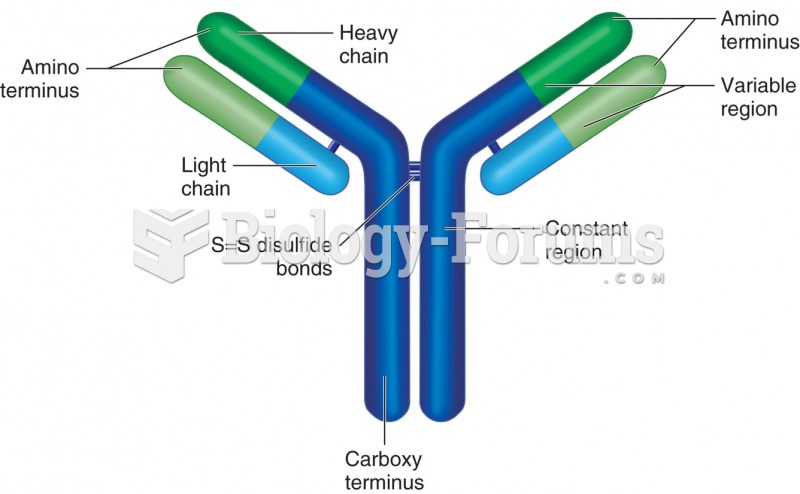 Schematic of an IgG molecule: Two heavy and 2 light chains are linked together by disulfide bonds. ...
Schematic of an IgG molecule: Two heavy and 2 light chains are linked together by disulfide bonds. ...