|
|
|
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
Although puberty usually occurs in the early teenage years, the world's youngest parents were two Chinese children who had their first baby when they were 8 and 9 years of age.
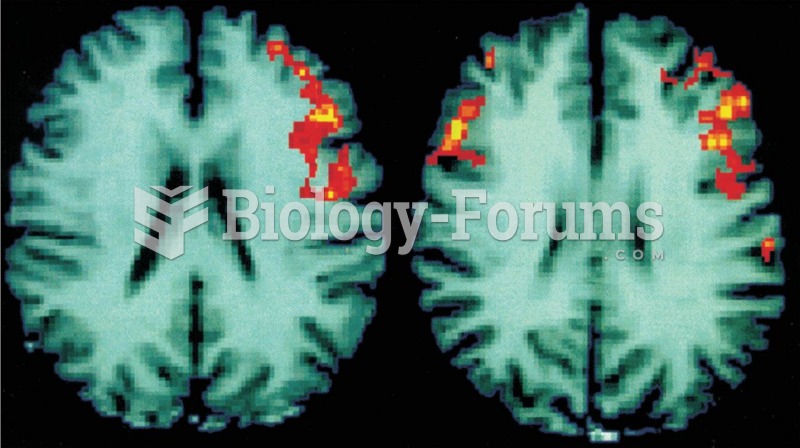
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.