|
|
|
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
 Uranus revolves around the Sun once every 84 Earth years. Its average distance from the Sun is rough
Uranus revolves around the Sun once every 84 Earth years. Its average distance from the Sun is rough
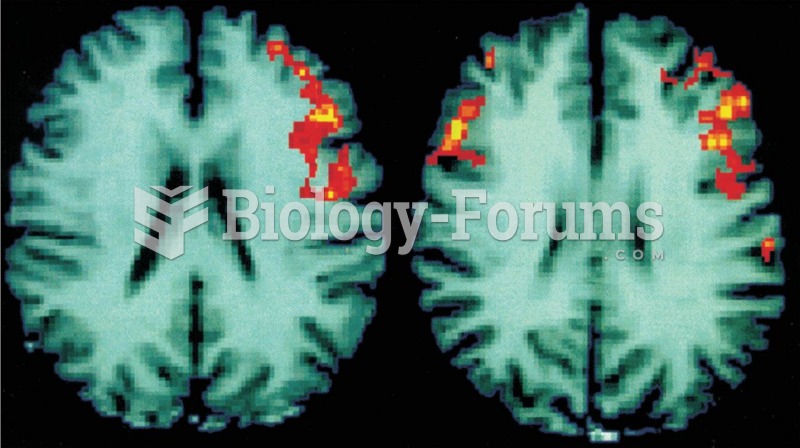 Functional MRI Scans These scans of human brains show localized average increases in neural activity
Functional MRI Scans These scans of human brains show localized average increases in neural activity