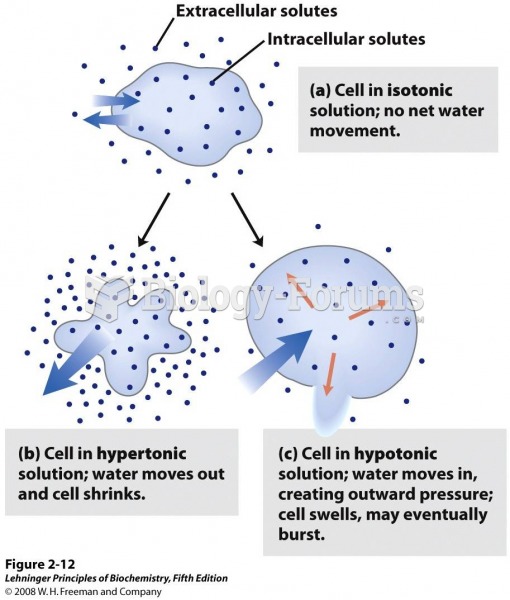
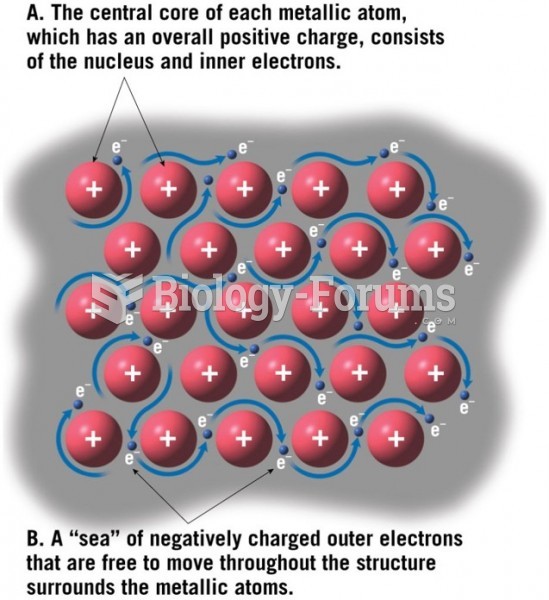
|
|
|
Did you know?
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Did you know?
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.