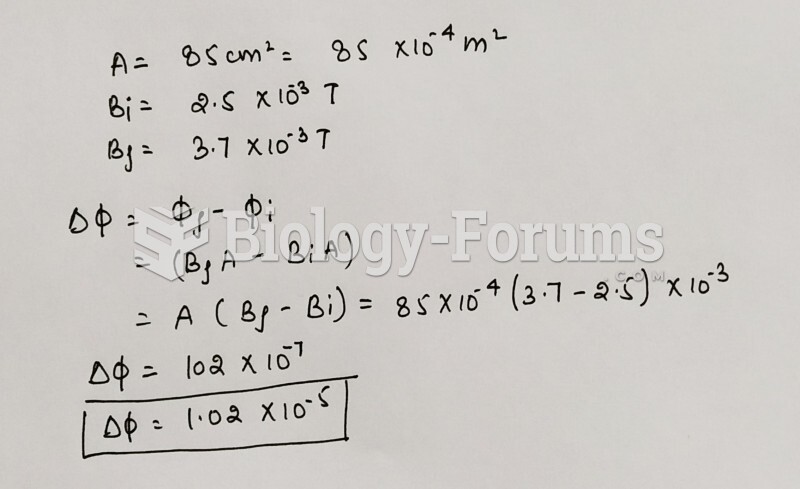
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
Did you know?
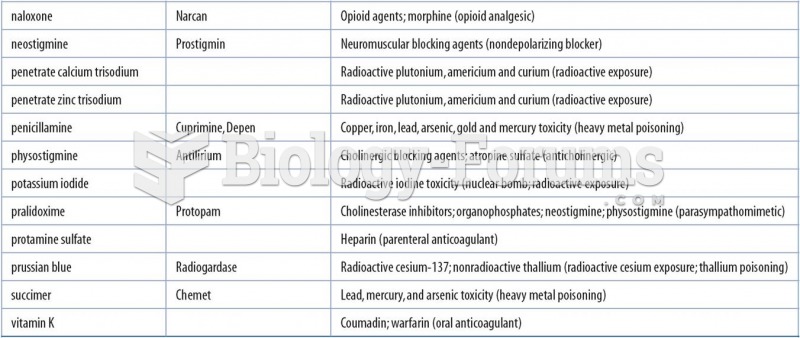
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Everyone has one nostril that is larger than the other.